Abstract
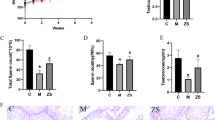
Tripterygium glycosides (TG) can seriously damage male reproductive function, and the reproductive system is difficult to restore after stopping the administration of TG in male rats. Zinc (Zn) is one of the most important trace elements in the human body and plays an important role in maintaining male fertility. The aim of this study was to investigate whether zinc supplementation could improve the testicular reproductive damage induced by TG toxicity in rats and to investigate its mechanism of action. The results showed that zinc sulfate (ZnSO4) could improve testicular tissue structure and semen parameters, promote testosterone synthesis, increase zinc-containing enzyme activity, increase zinc concentration in serum and testicular tissues, and maintain zinc homeostasis in male rats induced by TG toxicity. Zinc supplementation activated relevant signalling molecules in the KEAP1-NRF2/ARE pathway and alleviated TG-induced oxidative stress. Therefore, this study concluded that zinc supplementation could improve reproductive damage by regulating zinc homeostasis and the expression of genes related to oxidative stress.






Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
(2015) Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 103: e18-e25. https://doi.org/10.1016/j.fertnstert.2014.12.103
Abdella AM, Elabed BH, Bakhiet AO, Gadir WSA, Adam SEI (2011) In vivo study on lead, cadmium, and zinc supplementations on spermatogenesis in albino rats. J Pharmacol Toxicol 2:141–148. https://doi.org/10.3923/jpt.2011.141.148
Adelakun SA, Ogunlade B, Fidelis OP, Omotoso OD (2022) Protective effect of nutritional supplementation of zinc-sulfate against cisplatin-induced spermatogonial and testicular dysfunctions in adult male Sprague-Dawley rats. Endocr Metab Sci 6:100116. https://doi.org/10.1016/j.endmts.2021.100116
Agarwal A, Baskaran S, Parekh N, Cho CL, Henkel R, Vij S, Arafa M, Panner SM, Shah R (2021) Male infertility. Lancet 397:319–333. https://doi.org/10.1016/S0140-6736(20)32667-2
Akintoye OO, Ajibare AJ, Folawiyo MA, Jimoh-Abdulghaffaar HO, Asuku A, Owolabi GA, Babalola KT (2023) Zinc supplement reverses short-term memory deficit in sodium benzoate-induced neurotoxicity in male Wistar rats by enhancing anti-oxidative capacity via Nrf 2 up-regulation. Behav Brain Res 437:114163. https://doi.org/10.1016/j.bbr.2022.114163
An Q, Zhang K, Fu L, Guo Y, Zhang C, Ge Z, Ma J, Gu Y, Zuo L (2020) The impact of exogenous testosterone supplementation on spermatogenesis in a rat model of oligoasthenospermia. Int J Clin Exp Pathol 13:1287–1299
Baltaci AK, Yuce K (2018) Zinc transporter proteins. Neurochem Res 43:517–530. https://doi.org/10.1007/s11064-017-2454-y
Bao B, Yang Z, Deng S, Dai H, Feng J, Meng F, Li H, Wang J (2022) Zuogui Wan improves spermatogenesis of GC1-spg cells through modulating AR-related pathways. Andrologia 54:e14407. https://doi.org/10.1111/and.14407
Zhang C, Wang X, Hu X, Cui Y, Tong J, Wang X (2008) Study on the suppression of the spermatogenesis in rats by Tripterygium wilfordii. J Reprod Med 2:118–122
Chen WQ, Wang B, Ding CF, Wan LY, Hu HM, Lv BD, Ma JX (2021) In vivo and in vitro protective effects of the Wuzi Yanzong pill against experimental spermatogenesis disorder by promoting germ cell proliferation and suppressing apoptosis. J Ethnopharmacol 280:114443. https://doi.org/10.1016/j.jep.2021.114443
Chu A, Holdaway C, Varma T, Petocz P, Samman S (2018) Lower serum zinc concentration despite higher dietary zinc intake in athletes: a systematic review and meta-analysis. Sports Med 48:327–336. https://doi.org/10.1007/s40279-017-0818-8
Chu Q, Chi ZH, Zhang X, Liang D, Wang X, Zhao Y, Zhang L, Zhang P (2016) A potential role for zinc transporter 7 in testosterone synthesis in mouse Leydig tumor cells. Int J Mol Med 37:1619–1626. https://doi.org/10.3892/ijmm.2016.2576
Croxford TP, McCormick NH, Kelleher SL (2011) Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. J Nutr 141:359–365. https://doi.org/10.3945/jn.110.131318
Dai Y, Sun L, Han S, Xu S, Wang L, Ding Y (2022) Proteomic study on the reproductive toxicity of tripterygium glycosides in rats. Front Pharmacol 13:888968. https://doi.org/10.3389/fphar.2022.888968
de Kretser DM (2004) Editorial: Is spermatogenic damage associated with Leydig cell dysfunction? J Clin Endocrinol Metab 89:3158–3160. https://doi.org/10.1210/jc.2004-0741
Fallah A, Mohammad-Hasani A, Colagar AH (2018) Zinc is an essential element for male fertility: a review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil 19:69–81
Foster M, Hancock D, Petocz P, Samman S (2011) Zinc transporter genes are coordinately expressed in men and women independently of dietary or plasma zinc. J Nutr 141:1195–1201. https://doi.org/10.3945/jn.111.140053
Gao HT, Di QN, Qian LL, Lu L, Li RX, Cao WX, Xu Q (2020) Zinc supplement ameliorates phthalates-induced reproductive toxicity in male rats. Chemosphere 246:125828. https://doi.org/10.1016/j.chemosphere.2020.125828
Guo X, Ye Y, Liu X, Sheng Y, Yu Y, Yang Y, Gu M, Lin R, Wang B, An L et al (2022) Effects of Agaricus blazei acidic polysaccharide on the aging of mice through keap1-Nrf2/ARE and MAPKs signal pathway. Electron J Biotechn 57:31–41. https://doi.org/10.1016/j.ejbt.2022.03.004
Han S, Dai Y, Sun L, Xing Y, Ding Y, Zhang X, Xu S (2023) Molecular mechanism of Cuscutae semen-radix rehmanniae praeparata in relieving reproductive injury of male rats induced with Tripterygium wilfordii multiglycosides: a tandem mass tag-based proteomics analysis. Front Pharmacol 14:1050907. https://doi.org/10.3389/fphar.2023.1050907
Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG et al (2003) Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene 22:6005–6012. https://doi.org/10.1038/sj.onc.1206797
Hunt CD, Johnson PE, Herbel J, Mullen LK (1992) Effects of dietary zinc depletion on seminal volume and zinc loss, serum testosterone concentrations, and sperm morphology in young men. Am J Clin Nutr 56:148–157. https://doi.org/10.1093/ajcn/56.1.148
Jawad HM (Zinc sulfate treatment of secondary male infertility associated with positive serum and seminal plasma anti-sperm antibody test. https://doi.org/10.1016/j.mefs.2012.09.005
Jianmin G, Yuanqiang H, Xialing L, Zhong H, Chun L, Wei Y (2018) Dynamic changes and mechanism of reproductive system injury induced by tripterygium glycosides in male SD rats. Chin J Pharmacol Toxicol 32:469–476
Jiao W, Sun J, Zhang X, An Q, Fu L, Xu W, Xie H, Tang X, Liu J, Hu W et al (2021) Improvement of Qilin pills on male reproductive function in tripterygium glycoside-induced oligoasthenospermia in rats. Andrologia 53:e13923. https://doi.org/10.1111/and.13923
Jing X, Cheng W, Guo S, Zou Y, Zhang T, He L (2017) Toxic effects of Tripterygium wilfordii Hook F on the reproductive system of adolescent male rats. Biomed Pharmacother 95:1338–1345. https://doi.org/10.1016/j.biopha.2017.09.038
Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH et al (2012) Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. Bmj Open 2. https://doi.org/10.1136/bmjopen-2012-000990
Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T (2016) Repression of the antioxidant NRF2 pathway in premature aging. Cell 165:1361–1374. https://doi.org/10.1016/j.cell.2016.05.017
Lu QX (1990) Effect of glycosides of Tripterygium wilfordii Hook on the reproductive system and major organs of male rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 12:203–207
Ma B, Qi H, Li J, Xu H, Chi B, Zhu J, Yu L, An G, Zhang Q (2015) Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology 336:84–95. https://doi.org/10.1016/j.tox.2015.07.008
Ma J, Bi J, Sun B, Li H, Li Y, Wang S (2023) Zinc improves semen parameters in high-fat diet-induced male rats by regulating the expression of LncRNA in testis tissue. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03550-7
Mahmoud AM, Germoush MO, Alotaibi MF, Hussein OE (2017) Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother 86:297–306. https://doi.org/10.1016/j.biopha.2016.12.047
Maremanda KP, Srivalliputturu SB, Jena G (2020) Zinc deficient diet exacerbates the testicular and epididymal damage in type 2 diabetic rat: Studies on oxidative stress-related mechanisms. Reprod Biol 20:191–201. https://doi.org/10.1016/j.repbio.2020.03.002
Mazani M, Argani H, Rashtchizadeh N, Ghorbanihaghjo A, Hamdi A, Estiar MA, Nezami N (2013) Effects of zinc supplementation on antioxidant status and lipid peroxidation in hemodialysis patients. J Ren Nutr 23:180–184. https://doi.org/10.1053/j.jrn.2012.08.012
Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Rink L, Malavolta M (2006) Zinc homeostasis in aging: two elusive faces of the same “metal.” Rejuvenation Res 9:351–354. https://doi.org/10.1089/rej.2006.9.351
Özaslan M, Kılıç IH, Aytekin T, Güldür ME, Bozkurt AL (2014) Investigation of antioxidant effect of zinc biochemically and histopathologically in rats. Biotechnol Biotec Eq 2:136–143. https://doi.org/10.1080/13102818.2005.10817205
Punjani N, Kang C, Schlegel PN (2020) Clinical implications of Y chromosome microdeletions among infertile men. Best Pract Res Clin Endocrinol Metab 34:101471. https://doi.org/10.1016/j.beem.2020.101471
Rasmussen PE, Erb K, Westergaard LG, Laursen SB (1997) No evidence for decreasing semen quality in four birth cohorts of 1,055 Danish men born between 1950 and 1970. Fertil Steril 68:1059–1064. https://doi.org/10.1016/s0015-0282(97)00377-4
Siqi G, Junqi H, Jingshang W, Lei D, Yutian Z (2021) The mechanism of glycosides of tripterygiumwilfordii Hook. f. on reproductive toxicity of mice based on transcriptomics. Chin J Human Sex 30:1–5
Sun B, Ma J, Te L, Zuo X, Liu J, Li Y, Bi J, Wang S (2023) Zinc-deficient diet causes imbalance in zinc homeostasis and impaired autophagy and impairs semen quality in mice. Biol Trace Elem Res 201:2396–2406. https://doi.org/10.1007/s12011-022-03324-1
Teng L, Xianwen F, Sisi L, Guang Z (2018) Effect of tonifying kidney on repairing male reproductive injury by promoting the proliferation of spermatogonial stem cells. Asia-Pac Tradit Med 14:5–8
Tubek S (2007) Zinc supplementation or regulation of its homeostasis: advantages and threats. Biol Trace Elem Res 119:1–9. https://doi.org/10.1007/s12011-007-0043-7
Wang F, Li Y, Cao Y, Li C (2015) Zinc might prevent heat-induced hepatic injury by activating the Nrf2-antioxidant in mice. Biol Trace Elem Res 165:86–95. https://doi.org/10.1007/s12011-015-0228-4
Wang J, Miao M, Zhang Y, Liu R, Li X, Cui Y, Qu L (2015) Quercetin ameliorates liver injury induced with Tripterygium glycosides by reducing oxidative stress and inflammation. Can J Physiol Pharmacol 93:427–433. https://doi.org/10.1139/cjpp-2015-0038
Wenjiao L, Jing M, Yuiyu H, Shusong W (2020) Expressions of zinc transporters in gonads of diabetic male rats. Chin J Androl 34:7–12
Xie D, Li K, Ma T, Jiang H, Wang F, Huang M, Sheng Z, Xie Y (2022) Therapeutic effect and safety of Tripterygium glycosides combined with Western medicine on type 2 diabetic kidney disease: a meta-analysis. Clin Ther 44:246–256. https://doi.org/10.1016/j.clinthera.2021.12.006
Xu Y, Fan YF, Zhao Y, Lin N (2019) Overview of reproductive toxicity studies on Tripterygium wilfordii in recent 40 years. Zhongguo Zhong Yao Za Zhi 44:3406–3414. https://doi.org/10.19540/j.cnki.cjcmm.20190524.401
Xu Y, Lei H, Guan R, Gao Z, Li H, Wang L, Song W, Gao B, Xin Z (2014) Studies on the mechanism of testicular dysfunction in the early stage of a streptozotocin induced diabetic rat model. Biochem Biophys Res Commun 450:87–92. https://doi.org/10.1016/j.bbrc.2014.05.067
Xue M, Jiang ZZ, Wu T, Yan M, Liu JP, Mu XM, Su YW, Zhang LY (2011) Protective effects of tripterygium glycoside-loaded solid lipid nanoparticles on male reproductive toxicity in rats. Arzneimittelforschung 61:571–576. https://doi.org/10.1055/s-0031-1300555
Yang Y, Feng Y, Huang H, Cui L, Li F (2021) PM2.5 exposure induces reproductive injury through IRE1/JNK/autophagy signaling in male rats. Ecotoxicol Environ Saf 211:111924. https://doi.org/10.1016/j.ecoenv.2021.111924
Yanmei W, Jiazhou Y, Yufeng Z, Qingyan A, Naizhou M (2012) The effect of zinc on male reproductive system and testicular function. Shandong Medicine 52:93–94
Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234. https://doi.org/10.1016/s0300-483x(02)00049-5
Yuzhen L, Ling W, Jinxia G, Jianmao C, Hongting C, Qiru S (2019) Effects of arsenic on spermatogenesis and serum testosterone concentration in male rats. J Ningxia Med Univ 41:897–900. https://doi.org/10.16050/j.cnki.issn1674-6309.2019.09.007
Zhang K, Fu L, An Q, Hu W, Liu J, Tang X, Ding Y, Lu W, Liang X, Shang X et al (2020) Effects of Qilin pills on spermatogenesis, reproductive hormones, oxidative stress, and the TSSK2 gene in a rat model of oligoasthenospermia. BMC Complement Med Ther 20:42. https://doi.org/10.1186/s12906-019-2799-7
Zhang TY, Wu RY, Zhao Y, Xu CS, Zhang WD, Ge W, Liu J, Sun ZY, Zou SH, Shen W (2018) Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways. Toxicol Appl Pharmacol 340:49–57. https://doi.org/10.1016/j.taap.2017.12.011
Zhang X, Guan T, Yang B, Chi Z, Wang ZY, Gu HF (2018) A novel role for zinc transporter 8 in the facilitation of zinc accumulation and regulation of testosterone synthesis in Leydig cells of human and mouse testicles. Metabolism 88:40–50. https://doi.org/10.1016/j.metabol.2018.09.002
Zhou X, Zhang GW, Liang WN, Li YZ, Xu S, Yan LL, Li HJ, Shang XJ (2021) Impact of moriamin forte on testicular and epididymal damage in rats with oligoasthenospermia. Evid Based Complement Alternat Med 2021:4059248. https://doi.org/10.1155/2021/4059248
Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ (2005) Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol 166:1681–1690. https://doi.org/10.1016/S0002-9440(10)62478-9
Zhu X, Yu C, Wu W, Shi L, Jiang C, Wang L, Ding Z, Liu Y (2022) Zinc transporter ZIP12 maintains zinc homeostasis and protects spermatogonia from oxidative stress during spermatogenesis. Reprod Biol Endocrinol 20:17. https://doi.org/10.1186/s12958-022-00893-7
Zini A, Schlegel PN (2003) Effect of hormonal manipulation on mRNA expression of antioxidant enzymes in the rat testis. J Urol 169:767–771. https://doi.org/10.1097/01.ju.0000049915.87039.f3
Funding
This work was supported by S&T Program of Hebei (Grant No. 203777107D); Hebei Natural Science Foundation (Grant No.H2022314001);
Science and Technology Department of Hebei Province (project number: 22567604D).
Author information
Authors and Affiliations
Contributions
Weiguang Lian raised experimental animals. He Tan and Bo Sun performed the experiments. Jiajie Bi analyzed the data. Yingxian Zhen collated the data. Jing Ma and Jiajie Bi wrote the manuscript. Jing Ma and Shusong Wang conceived the idea, designed the study, and revised the manuscript. Shusong Wang, Weiguang Lian, and Yingxian Zhen collected the funds. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All experimental procedures were approved by the ethics committee of the Hebei Institute of Reproductive Health Science and Technology.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, J., Tan, H., Bi, J. et al. Zinc Ameliorates Tripterygium Glycosides–Induced Reproductive Impairment in Male Rats by Regulating Zinc Homeostasis and Expression of Oxidative Stress–Related Genes. Biol Trace Elem Res 202, 2111–2123 (2024). https://doi.org/10.1007/s12011-023-03815-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03815-9