Abstract
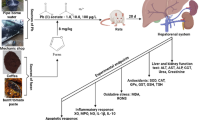
Drinking water polluted by heavy metals has the potential to expose delicate biological systems to a range of health issues. This study embraced the health risks that may arise from subchronic exposure of thirty-four male Wistar rats to nickel (Ni)-cadmium (Cd)-contaminated water. It was done by using the Box-Behnken design (BBD) with three treatment factors (Ni and Cd doses at 50–150 mg/L and exposure at 14–21-28 days) at a single alpha level, resulting in seventeen experimental combinations. Responses such as serum creatinine (CREA) level, blood urea nitrogen (BUN) level, BUN/CREA ratio (BCR), aspartate and alanine aminotransferases (AST and ALT) activities, and the De Ritis ratio (DRR), as well as malondialdehyde (MDA) level, catalase (CAT), and superoxide dismutase (SOD) activities, were evaluated. The results revealed that these pollutants jointly caused hepatocellular damage by raising AST and ALT activities and renal dysfunction by increasing CREA and BUN levels in Wistar rats’ sera (p < 0.05). These outcomes were further supported by BCR and DRR values beyond 1. In rats’ hepatocytes and renal tissues, synergistic interactions of these metals resulted in higher MDA levels and significant impairments of CAT and SOD activities (p < 0.05). In order to accurately forecast the effects on the responses, the study generated seven acceptable regression models (p < 0.05) with r-squared values of > 80% at no discernible lack of fit (p > 0.05). The findings hereby demonstrated that Wistar rats exposed to these pollutants at varied doses had increased risks of developing liver cirrhosis and azotemia marked by metabolic stress.










Similar content being viewed by others
Data Availability
Authors are bound to issues of experimental data on rational grounds, but relevant data are already made available in the article and in the supplementary file.
Code Availability
The adoption of design expert software (version 13.0) was affirmed in the study for its experimentation, data analyses, models’ developments, graphical fittings, and critical inferences.
References
WHO (World Health Organization) (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
USEPA (U.S. Environmental Protection Agency) (2015) Regulated drinking water contaminants. http://www.epa.gov/dwstandardsregulations. Accessed Mar 2023
Jamshaid M, Khan AA, Ahmed K, Saleem M (2018) Heavy metal in drinking water its effect on human health and its treatment techniques-a review. Int J Biosci 12(4):223–240. https://doi.org/10.12692/ijb/12.4.223-240
Nastasescu V, Mititelu M, Goumenou M, Docea OA, Renieri E, Udeanu DI, Oprea E, Arsene AL, Dinu-Pîrvu CE, Ghica M (2020) Heavy metal and pesticide levels in dairy products: evaluation of human health risk. Food Chem Toxicol 146:111844. http://refhub.elsevier.com/S2214-7500(22)00094-4/sbref2. Accessed Feb 2023
Ugwu CE, Maduka IC, Suru SM, Anakwuo IA (2022) Human health risk assessment of heavy metals in drinking water sources in three senatorial districts of Anambra State, Nigeria. Toxicol Reports 9:869–875. https://doi.org/10.1016/j.toxrep.2022.04.011
World Bank (2016) The World Bank data: country and lending groups. http://data.worldbank.org/about/country-and-lending-groups#Low_income. Accessed Mar 2023
WHO (World Health Organization) (2021) Nickel in drinking-water. Background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva; 2021 (WHO/HEP/ECH/WSH/2021.6). Licence: CC BY-NC-SA 3.0 IGO
Apiamu A, Asagba SO, Tonukari NJ (2019) Role of Anthocleista vogelii in serum antioxidant defence system in cadmium-induced oxidative stress in Wistar rats. Beni-Suef Univ J Basic Appl Sci 8(12):1–13. https://doi.org/10.1186/s43088-019-0012-1
Health Canada (2020) Guidelines for Canadian drinking water quality: Guideline technical document – Cadmium. http://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-is
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxicological profile for Nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. http://www.atsdr.cdc.gov/
OEHHA (Office of Environmental Health Hazard Assessment) (2012) Nickel reference exposure levels. Nickel and nickel compounds. Nickel oxide. Reference exposure levels (RELs). https://oehha.ca.gov/media/downloads/crnr/032312nirelfinal.pdf
Bonfim DJP, Garcia FM, Laposy CB, Giuffrida R, Nai GA, Net HB (2021) Influence of water pH in the hepato- and nephrotoxicity of chronic cadmium poisoning in Wistar rats. Res Soc Dev 10(9):e12210917753. https://doi.org/10.33448/rsd-v10i9.17753
Das SC, Al-Naemi HA (2019) Cadmium toxicity: oxidative stress, inflammation and tissue injury. Occup Dis Environ Med 7:144–163. https://doi.org/10.4236/odem.2019.74012
Asagba SO, Eriyamremu GE, Igberaese ME (2008) Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish Physiol Biochem 34:61–69. https://doi.org/10.1007/s10695-007-9147-4
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940. https://doi.org/10.1007/s10534-010-9329-x
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17:3782–3806. https://doi.org/10.3390/ijerph17113782
Boxton S, Garman E, Heim KE, Lyons-Darden T, Schlekat CE, Taylor MD, Oller AR (2019) Concise review of nickel human health toxicology and ecotoxicology. MDPI Iorg 7:89–127. https://doi.org/10.3390/inorganics7070089
Das KK, Reddy RC, Bagoji IB, Das S, Bagali S, Mullur L, Khodnapur JP, Biradar MS (2018) Primary concept of nickel toxicity – an overview. J Basic Clin Physiol Pharmacol 1–12. https://doi.org/10.1515/jbscpp-2017-0171
De Brouwere K, Buekers J, Cornelis C, Schlekat CE, Oller AR (2012) Assessment of indirect human exposure to environmental sources of nickel: oral exposure and risk characterization for systemic effects. Sci Total Environ 419:25–36. https://doi.org/10.1016/j.scitotenv.2011.12.049
IARC (International Agency for Research on Cancer) (2017) IARC monographs on the evaluation of carcinogenic risks to humans: nickel and nickel compounds monograph. WHO Press: Geneva, Switzerland 100C:169–218
EFSA (European Food Safety Agency), (2015) Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water, EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 13:4002
Thyssen JP (2010) Menné T (2010) Metal allergy—a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 23:309–318. https://doi.org/10.1021/tx9002726
Saito M, Arakaki R, Yamada A, Tsunematsu T, Kudo Y, Ishimaru N (2016) Molecular mechanisms of nickel allergy. Int J Mol Sci 17:202. https://doi.org/10.3390/ijms17020202
Das KK, Dasgupta S (2002) Effect of nickel sulfate on testicular steroidogenesis in rats during protein restriction. Environ Health Perspect 110:923–926
Tikare SN, Gupta AD, Dhundasi SA, Das KK (2008) Effect of antioxidants L-ascorbic acid and alpha-tocopherol supplementation in nickel exposed hyperglycemic rats. J Basic Clin Physiol Pharmacol 19:89–10
Samir D, Zine K (2013) Preventive effect of zinc on nickel-induced oxidative liver injury in rats. Afr J Biotechnol 12(5):7112–7119. https://doi.org/10.5897/AJB2013.12962
Novelli ELB, Hernandes RT, Novelli-Filho JLVB, Babbosa LL (1998) Differential/combined effect of water contamination with cadmium and nickel on tissues of rats. Environ Pollution 103:295–300
Apiamu A, Asagba SO (2021) Zinc-cadmium interactions instigated antagonistic alterations in lipid peroxidation, ascorbate peroxidase activity and chlorophyll synthesis in Phaseolus vulgaris leaves. Sci Afr 11:e00688. https://doi.org/10.1016/j.sciaf.2020.e00688
Said KAM, Amin MAM (2015) Overview on the response surface methodology in extract processes. J Appl Sci Process Eng 2(1):8–17
Apiamu A, Osawaru SU, Asagba SO, Evuen UF, Achuba FI (2021) Exposure of African catfish (Clarias gariepinus) to lead and zinc modulates membrane-bound transport protein: a plausible effect on Na+/K+-ATPase activity. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-03005-5
Asagba SO, Apiamu A, Enokpe FE (2019) Effects of nickel toxicity on the indices of germination and Ca2+ ATPase activity in cowpea plant (Vigna unguiculata). JASEM 23(6):1147–1152
Lamtai M, Chaibat J, Ouakki S, Berkiks I, Riti E, EI-Hessni A, Mesfioui A, Hbibi AT, Ahyayauch H, Essamri A, Ouichou A (2018) Effect of chronic administration of cadmium on anxiety-like, depression-like and memory deficits in male and female rats: possible involvement of oxidative stress mechanism. J Behav Brain Sci 8: 240-26.https://doi.org/10.4236/jbbs.2018.85016
Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamate oxaloacetate and glutamic pyruvate transaminase. Am J Clin Pathol 28:56–58
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Annals Chem 39:971–974
Prabhakara PV, Reddya UA, Singha SP (2012) Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appli Toxicol 32:436–445
Gavali Y, Deore D, Surwase SP, Zingade U (2013) Study of the serum superoxide dismutase levels in smoking and non-smoking patience with COPD. Int J Recent Trends Sci technol 5:121–126
Ezedom T, Asagba SO, Tonukari NJ (2020) Toxicological effects of the concurrent administration of cadmium and arsenic through the food chain on the liver and kidney of rats. J Basic Appl Zool 81:16–28. https://doi.org/10.1186/s41936-020-00146-2
Singh K (2013) Evaluation and interpretation of biomarkers of liver diseases. Int J Res Health Sci 1(3):213–223
Gao F, Chen C, Lu J, Zheng J, Ma X, Yuan X, Huo K, Ha J (2017) De Ritis ratio (AST/ALT) as an independent predictor of poor outcome in patients with acute ischemic stroke. Neuropsychiatr Dis Treat 13:1551–1557. https://doi.org/10.2147/NDT.S139316
Apiamu A, Asagba SO, Tonukari NJ (2018) Reporting the toxicological profile of methanolic leaf extract of Anthocleista vogelii planch on Wistar rats. JASEM 22(6):887–894. https://doi.org/10.4314/jasem.v22i6.8
Lumongga F, Anggraeni R (2016) Correlation between aspartate aminotransferase/alanine transferase ratio (AST/ALT ratio) and stage of liver fibrosis in patients with chronic hepatitis. Adv Health Sci Res 1:72–75
Aberg F, Danford CJ, Thiele M, Talbäck M, Rasmussen DN, Jiang ZG et al (2021) A dynamic aspartate-to-alanine aminotransferase ratio provides valid predictions of incident severe liver disease. Hepatol Comm 5:1021–1035
Griffin BR, Faubel S, Edelstein CL (2019) Biomarkers of drug-induced kidney toxicity. Ther Drug Monit 41(2):213–226. https://doi.org/10.1097/FTD.0000000000000589
Pócsi I, Dockrell ME, Price RG (2022) Nephrotoxic biomarkers with specific indications for metallic pollutants: implications for environmental health. Biomark Insights 17:1–13. https://doi.org/10.1177/11772719221111882
Suganya R, Priya S, Rajini S, Rajagopalan B (2016) A study to evaluate the role of bun/creatinine ratio as a discriminator factor in azotemia. Int J Pharm Sci Rev Res 40(1):131–134
Asagba SO, Eriyamremu GE, Emudainohwo JOT, Okoro I (2010) Oxidative enzymes in tissues of the catfish (Clarias gariepinus) exposed to varying levels of cadmium. Environ 30:260–266
Gagné F (2014) Oxidative stress. In: Biochemical ecotoxicology, Elsevier, Amsterdam, 103–115. https://doi.org/10.1016/B978-0-12-411604-7.00006-4
Saleh RM, Awadin WF (2017) Biochemical and histopathological changes of subacute cadmium intoxication in male rats. Environ Sci Pollution Res 24:25475–25481. https://doi.org/10.1007/s11356-017-0348-9
Ramesh G, Madhuri D, Lakshman M, Reddy AG (2018) Histopathological changes in bone marrow induced by lead and cadmium alone and combined exposure in male Wistar rats. J Entomol Zool St 6:3035–3037
Peijnenburg WJGM, Vijver MG (2007) Metal-specific interactions at the interface of chemistry and biology. Pure Appl Chem 79(12):2351–2366. https://doi.org/10.1351/pac200779122351
Sauliutė G, Gintaras G (2015) Heavy metal interactions during accumulation via direct route in fish: a review. Zool Ecol 25(1):77–86. https://doi.org/10.1080/21658005.2015.1009734
Kanmani P, Karthik S, Aravind J, Kumaresan K (2013) The use of response surface methodology as a statistical tool for media optimization in lipase production from the dairy effluent isolate Fusarium solani. ISRN Biotechnol 2013:1–8. https://doi.org/10.5402/2013/528708
Jha P, Das AJ, Deka SC (2018) Optimisation of fermentation process for production of black rice wine and evaluation of its phenolic and volatile compounds. J Inst Brew 124:485–491. https://doi.org/10.1002/jib.504
Acknowledgements
This collaborative investigation would not have been possible without the provision of research facilities and enabling environments. Therefore, we are deeply thankful to the departments of respective universities of authors, who contributed immensely to the success of this article.
Author information
Authors and Affiliations
Contributions
The successful completion of the manuscript was made possible through the notable inputs of the authors: AA, OJA, UFE, HEK, EDK, AAA, GU, and SOA participated in all sections of the study, starting from conceptualization, research design, drafting of proposal, provision of research materials and apparatuses, laboratory investigation, analyses of experimental data, presentation and interpretation of results, drafting and editing of manuscript. Absolute consent by all authors was acknowledged with respect to submission of the manuscript to this journal.
Corresponding author
Ethics declarations
Ethics Approval
Sequel to ethics of care and handling of experimental animals, the local research ethics committee gave her approval (REC/FOS/2023/02), in line with international protocols.
Consent to Participate
Not applicable.
Consent for Publication
We affirm that no form of data from individuals or group of persons is associated with the publication of the manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Apiamu, A., Avwioroko, O.J., Evuen, U.F. et al. Exposure to Nickel–Cadmium Contamination of Drinking Water Culminates in Liver Cirrhosis, Renal Azotemia, and Metabolic Stress in Rats. Biol Trace Elem Res 202, 1628–1643 (2024). https://doi.org/10.1007/s12011-023-03777-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03777-y