Abstract
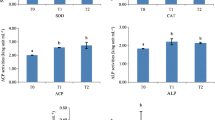
Zinc is an essential micronutrient for organisms involved in regulating various biological processes. This study evaluated the effects of dietary zinc on growth performance, digestive enzyme activities, antioxidant status, and immune responses of sea cucumber Apostichopus japonicus. Five experimental diets were formulated with graded levels of zinc (0, 20, 40, 60, and 80 mg/kg, respectively), and the actual dietary zinc values were 31.4, 51.0, 68.2, 91.9, and 110.8 mg/kg diet, respectively. Sea cucumbers were fed with diets for 2 months. The results showed the growth performance, amylase, and trypsin activities of sea cucumber increased significantly with zinc supplementation, and the best growth performance and enzyme activities were observed at 40 mg/kg zinc diet. Zinc supplementation significantly increased activities of superoxide dismutase, catalase, anti-superoxide anion, and inhibiting hydroxyl radical, while significantly reduced the malondialdehyde content. Furthermore, the higher zinc supplementation levels resulted in significantly upregulated immune-related genes of hsp90, p105, rel, and lsz, suggesting that excessive zinc caused oxidative stress. The broken-line regression analysis of specific growth rate indicated dietary zinc requirement in juvenile sea cucumber was ~ 66.3 mg/kg diet. Overall, dietary zinc contributes to the growth and immune resistance of juvenile sea cucumber, and our study will provide insights into the rational use of dietary zinc in aquaculture.




Similar content being viewed by others
Data Availability
Data and materials supporting the results are available upon reasonable request from the corresponding author.
References
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods-a review. Mar Drugs 9:1761–1805. https://doi.org/10.3390/md9101761
Bureau of Fisheries of the Ministry of Agriculture (2021) Fisheries Statistical Yearbook of China. China Agricultural Press, Beijing
Gutteridge JMC, Halliwell B (1992) Comments on review of free-radicals in biology and medicine. Free Radical Biol Med 12:93–95. https://doi.org/10.1016/0891-5849(92)90062-l
Ren Y, Liu W, Pearce CM (2018) Effects of stocking density, ration and temperature on growth, survival and metamorphosis of auricularia larvae of the California sea cucumber, Parastichopus californicus. Aquac Res 49:517–525. https://doi.org/10.1111/are.13482
Bray TM, Bettger WJ (1990) The physiological role zinc as an antioxidant. Free Radical Biol Med 8:281–291. https://doi.org/10.1016/0891-5849(90)90076-u
Dawood MAO, Alagawany M, Sewilam H (2022) The role of zinc microelement in aquaculture: a review. Biol Trace Elem Res 200:3841–3853. https://doi.org/10.1007/s12011-021-02958-x
Feng L, Tan LN, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr 17:E875–E882. https://doi.org/10.1111/j.1365-2095.2011.00858.x
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151:185–207. https://doi.org/10.1016/s0044-8486(96)01503-7
Shukry M, Albogami S, Gewaily M, Amer AA, Soliman AA, Alsaiad SM, El-Shehawi AM, Dawood MAO (2022) Growth performance, antioxidative capacity, and intestinal histomorphology of grey mullet (Liza ramada)-fed dietary zinc nanoparticles. Biol Trace Elem Res 200:2406–2415. https://doi.org/10.1007/s12011-021-02844-6
Jiang M, Wu F, Huang F, Wen H, Liu W, Tian J, Yang C, Wang W (2016) Effects of dietary Zn on growth performance, antioxidant responses, and sperm motility of adult blunt snout bream, Megalobrama amblycephala. Aquaculture 464:121–128. https://doi.org/10.1016/j.aquaculture.2016.06.025
Tan LN, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquac Nutr 17:338–345. https://doi.org/10.1111/j.1365-2095.2010.00793.x
Mohammady EY, Soaudy MR, Abdel-Rahman A, Abdel-Tawwab M, Hassaan MS (2021) Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia, Oreochromis niloticus. Aquaculture 532. https://doi.org/10.1016/j.aquaculture.2020.736006
Tan BP, Mai KS (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 192:67–84. https://doi.org/10.1016/s0044-8486(00)00435-x
Yuan Y, Luo J, Zhu T, Jin M, Jiao L, Sun P, Ward TL, Ji F, Xu G, Zhou Q (2020) Alteration of growth performance, meat quality, antioxidant and immune capacity of juvenile Litopenaeus vannamei in response to different dietary dosage forms of zinc: comparative advantages of zinc amino acid complex. Aquaculture 522. https://doi.org/10.1016/j.aquaculture.2020.735120
Kishawy ATY, Roushdy EM, Hassan FAM, Mohammed HA, Abdelhakim TMN (2020) Comparing the effect of diet supplementation with different zinc sources and levels on growth performance, immune response and antioxidant activity of tilapia, Oreochromis niloticus. Aquac Nutr 26:1926–1942. https://doi.org/10.1111/anu.13135
Maage A, Julshamn K (1993) Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture 117:179–191. https://doi.org/10.1016/0044-8486(93)90134-k
Shiau SY, Jiang LC (2006) Dietary zinc requirements of grass shrimp, Penaeus monodon, and effects on immune responses. Aquaculture 254:476–482. https://doi.org/10.1016/j.aquaculture.2005.10.033
Zhang M, Huang Y, Li Y, Cai M, Zhao Y (2021) The effects of dietary zinc on growth, immunity and reproductive performance of female Macrobrachium nipponense prawn. Aquac Res 52:1585–1593. https://doi.org/10.1111/are.15010
Shi B, Wang T, Zeng Z, Zhou L, You W, Ke C (2019) The role of copper and zinc accumulation in defense against bacterial pathogen in the Fujian oyster (Crassostrea angulata). Fish Shellfish Immunol 92:72–82. https://doi.org/10.1016/j.fsi.2019.05.049
Yu H, Gao Q, Dong S, Hou Y, Wen B (2016) Change of digestive physiology in sea cucumber Apostichopus japonicus (Selenka) induced by corn kernels meal and soybean meal in diets. J Ocean Univ China 15:697–703. https://doi.org/10.1007/s11802-016-2985-x
AOAC (2006) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Arlington, VA, USA
Chen J, Ren Y, Wang G, Xia B, Li Y (2018) Dietary supplementation of biofloc influences growth performance, physiological stress, antioxidant status and immune response of juvenile sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immunol 72:143–152. https://doi.org/10.1016/j.fsi.2017.10.061
Mei Y, Tian Y, Gao Q, Dong S, Li X, Xu Y (2022) Effects of different stocking densities on the CO2 fluxes at water-air interface and the respiration metabolism in sea cucumber Apostichopus japonicus (Selenka). Front Mar Sci 9. https://doi.org/10.3389/fmars.2022.965700
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Mahboub HH, Shahin K, Zaglool AW, Roushdy EM, Ahmed SAA (2020) Efficacy of nano zinc oxide dietary supplements on growth performance, immunomodulation and disease resistance of African catfish Clarias gariepinus. Dis Aquat Org 142:147–160. https://doi.org/10.3354/dao03531
Moazenzadeh K, Islami HR, Zamini A, Soltani M (2018) Effects of dietary zinc level on performance, zinc status, tissue composition and enzyme activities of juvenile Siberian sturgeon, Acipenser baerii (Brandt 1869). Aquac Nutr 24:1330–1339. https://doi.org/10.1111/anu.12670
Puar P, Niyogi S, Kwong RWM (2020) Regulation of metal homeostasis and zinc transporters in early-life stage zebrafish following sublethal waterborne zinc exposure. Aquat Toxic 225. https://doi.org/10.1016/j.aquatox.2020.105524
Ma S, Shu X, Wang WX (2022) Multi-omics reveals the regulatory mechanisms of zinc exposure on the intestine-liver axis of golden pompano Trachinotus ovatus. Sci Total Environ 816. https://doi.org/10.1016/j.scitotenv.2021.151497
Li B, Wang L, Wang J, Sun Y, Wang S, Jiang L, Huang B (2020) Requirement of vitamin E of growing sea cucumber Apostichopus japonicus Selenka. Aquac Res 51:1284–1292. https://doi.org/10.1111/are.14479
Wang X, Jiang J, Guan X, Zhao Z, Dong Y, Wang J, Li S, Jiang B, Liu G, Sun H, Gao S, Jiang P, Wang X, Zhou Z (2021) Effects of Lactobacillus acidophilus and tussah immunoreactive substances on disease resistance of sea cucumber (Apostichopus japonicus) against Vibrio splendidus. Aquac Res 52:4601–4612. https://doi.org/10.1111/are.15293
Li JS, Li JL, Wu TT (2007) The effects of copper, iron and zinc on digestive enzyme activity in the hybrid tilapia Oreochromis niloticus (L.) x Oreochromis aureus (Steindachner). J Fish Biol 71:1788–1798. https://doi.org/10.1111/j.1095-8649.2007.01643.x
Muralisankar T, Bhavan PS, Radhakrishnan S, Seenivasan C, Srinivasan V, Santhanam P (2015) Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 448:98–104. https://doi.org/10.1016/j.aquaculture.2015.05.045
Liao ML, Ren TJ, Chen W, Han YZ, Liu CM, Jiang ZQ, Wang FQ (2017) Effects of dietary lipid level on growth performance, body composition and digestive enzymes activity of juvenile sea cucumber, Apostichopus japonicus. Aquac Res 48:92–101. https://doi.org/10.1111/are.12864
Luo Z, Tan XY, Zheng JL, Chen QL, Liu CX (2011) Quantitative dietary zinc requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on hepatic intermediary metabolism and antioxidant responses. Aquaculture 319:150–155. https://doi.org/10.1016/j.aquaculture.2011.06.047
Hasnat A, Rani B, Kohli MPS, Chandraprakash G (2012) Zinc supplementation and its effect on thermal stress resistance in Carassius auratus fry. Isr J Aquacult-Bamidgeh 64:779–786
Nyska A, Kohen R (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. https://doi.org/10.1080/01926230290166724
Winston GW, Digiulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161. https://doi.org/10.1016/0166-445x(91)90033-6
Hidalgo MC, Exposito A, Palma JM, de la Higuera M (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34:183–193. https://doi.org/10.1016/s1357-2725(01)00105-4
Weissman L, De Souza-Pinto NC, Stevnsner T, Bohr VA (2007) DNA repair, mitochondria, and neurodegeneration. Neuroscience 145:1318–1329. https://doi.org/10.1016/j.neuroscience.2006.08.061
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biol Med 9:515–540. https://doi.org/10.1016/0891-5849(90)90131-2
Wallin RPA, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG (2002) Heat-shock proteins as activators of the innate immune system. Trends Immunol 23:130–135. https://doi.org/10.1016/s1471-4906(01)02168-8
Yang A, Zhou Z, Dong Y, Jiang B, Wang X, Chen Z, Guan X, Wang B, Sun D (2010) Expression of immune-related genes in embryos and larvae of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 29:839–845. https://doi.org/10.1016/j.fsi.2010.07.023
Wang QL, Yu SS, Qin CX, Dong SL, Dong YW (2014) Combined effects of acute thermal and hypo-osmotic stresses on osmolality and hsp70, hsp90 and sod expression in the sea cucumber Apostichopus japonicus Selenka. Aquacult Int 22:1149–1161. https://doi.org/10.1007/s10499-013-9734-6
Wang T, Sun Y, Jin L, Thacker P, Li S, Xu Y (2013) Aj-rel and Aj-p105, two evolutionary conserved NF-kappa B homologues in sea cucumber (Apostichopus japonicus) and their involvement in LPS induced immunity. Fish Shellfish Immunol 34:17–22. https://doi.org/10.1016/j.fsi.2012.09.006
Acknowledgements
The authors would like to express their sincere thanks to the staff of laboratory for their help and logistic support during the experiment.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Project Nos. 31672657).
Author information
Authors and Affiliations
Contributions
Yuling Xu, Qinfeng Gao, and Shulin Dong conceived and designed the experiments; Yaoping Mei, Xueqi Li, Kang Dong, and Zhao Li participated in the collection and determination of the experimental samples; Yuling Xu wrote the article; Qinfeng Gao and Zhishuai Hou revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were conducted in accordance with the guidelines and approval of the respective Animal Research and Ethics Committees of Ocean University of China (Permit Number: SCXY-B20230203). The field studies did not involve endangered or protected species.
Competing Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Gao, Q., Dong, S. et al. Effects of Dietary Zinc on Growth Performance, Digestive Enzyme Activities, Antioxidant Status, and Immune Responses of Sea Cucumber Apostichopus japonicus. Biol Trace Elem Res 202, 1767–1775 (2024). https://doi.org/10.1007/s12011-023-03766-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03766-1