Abstract
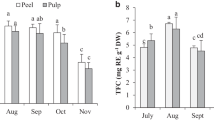
Grewia asiatica L. (phalsa) is a very prevalent berry in Pakistan and is consumed extensively as raw or in the form of juice. Here, for the first time, we assessed phalsa from Pakistan in terms of variations in macro and micro minerals, nutrients, and bio-active phyto-constituents including total phenolic and anthocyanin contents at different fruit developmental stages. It was found that the sugars in phalsa increased from D1 (small at the initial fruit setting stage) to D6 development stage (fully ripened fruit) where sugars at D5 (near to fully ripe) and D6 stages were many times greater than at D1, D2 (unripe close to full-size completion), D3 (close to semi ripe), and D4 stage (semi ripened and full-size attainment). Total acidity of was declined in all developmental stages, where the D1 stage displayed maximum and D6 with the lowest acidity. Ascorbic acid was decreased from D1 to D2 and then increased gradually from D3 to D5 stages. At the D6 stage, again a steep decline in ascorbic acid was observed. The total phenolics (mg gallic acid equivalents/100g) at stage D6 were higher (136.02 ± 1.17), whereas D1 being the lowermost in total phenolic content (79.89 ± 1.72). For anthocyanins (mg/100g), an increasing pattern of changes was observed in all stages of phalsa fruit where the D1 stage showed lower (13.97 ± 4.84) anthocyanin contents which then increased gradually at stage D2 (67.79 ± 6.73), but increased sharply at D3 (199.66 ± 4.90), D4 (211.02 ± 18.85), D5 (328.41 ±14.96) and D6 (532.30 ± 8.51) stages. A total of four anthocyanins such as cyanidin, delphidine-3-glucoside, pelargonidin, and malvidin in phalsa were identified using HPLC procedures, and a significant > 90 % DPPH inhibition in phalsa was observed at the D5 and D6 development stages. The macro and micro minerals including Ni, Zn, Fe, Ca, Cu, Mg, Na, P, and K contents were decreased from initial (D1) stage to the final (D6) development stage, while only Fe displayed an increasing trend from the initial to final fruit development stages (D1-D6). Conclusively, these findings could be of great interest for patients who are intended to consume phalsa as adjuvant therapy against diabetes and metabolic syndromes and other diseases involving reactive oxygen species with minimum metal toxicity.




Similar content being viewed by others
Data Availability
All the supporting data has been included in the manuscript.
Code Availability
Not applicable.
References
Gostner JM, Becker K, Ueberall F, Fuchs D (2015) The good and bad of antioxidant foods: an immunological perspective. Food Chem Toxicol 80:72–79. https://doi.org/10.1016/j.fct.2015.02.012
Alissa EM, Ferns GA (2017) Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr 57(9):1950–1962. https://doi.org/10.1080/10408398.2015.1040487
Nyanchoka MA, van Stuijvenberg ME, Tambe AB, Zuma MK, Mbhenyane XG (2022) Fruit and vegetable consumption patterns and risk of chronic diseases of lifestyle among University students in Kenya. Int J Environ Res Public Health 19:6965. https://doi.org/10.3390/ijerph19126965
Almeida MMB, Souza PHM, Arriaga AMC, Prado GMP, Magãlhaes CEC, Maia GA, de Limos TLG (2011) Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int 44:2155–2159. https://doi.org/10.1016/j.foodres.2011.03.051
Dhalaria R, Verma R, Kumar D, Puri S, Tapwal A, Kumar V, Nepovimova E, Kuca K (2020) Bioactive compounds of edible fruits with their anti-aging properties: a comprehensive review to prolong human life. Antioxidants 9(11):1123. https://doi.org/10.3390/antiox9111123
Gupta A, Kagliwal LD, Singhal RS (2013) Biotransformation of polyphenols for improved bioavailability and processing stability. Adv Food Nutr Res 69:183–217
Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F (2013) Novel insights into the pharmacology of flavonoids. Phytother Res 27:1588–1596. https://doi.org/10.1002/ptr.5023
Hamilton CA, Miller WH, Al-Benna S, Bronsan MJ, Drummond RD, McBride MW, Dominiczak AF (2004) Strategies to reduce oxidative stress in cardiovascular disease. Clin Sci 106:219–234. https://doi.org/10.1042/CS20030379
de Souza VR, Pereira PAP, da Silva TLT, de Oliveira Lima LC, Pio R, Queiroz F (2014) Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem 156:362–368. https://doi.org/10.1016/j.foodchem.2014.01.125
McKay DL, Chen CY, Oliver Zampariello CA, Blumberg JB (2015) Flvonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy adults. Food Chem 168:233–240. https://doi.org/10.1016/j.foodchem.2014.07.062
Pantelidis GE, Vasilakakis M, Manganaris GA, Gr D (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem 102:777–783. https://doi.org/10.1016/j.foodchem.2006.06.021
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Shah U, Shah R, Acharya S, Acharya N (2013) Novel anticancer agents from plant sources. Chin J Nat Med 11(1):16–23. https://doi.org/10.1016/S1875-5364(13)60002-3
Paul S (2015) Pharmacological actions and potential uses of Grewia asiatica: a review. Int J Appl Res 1:222–228
Koley TK, Khan Z, Oulkar D, Singh B, Bhatt BP, Banerjee K (2020) Profiling of polyphenols in phalsa (Grewia asiatica L) fruits based on liquid chromatography high resolution mass spectrometry. J Food Sci Technol 57(2):606–616. https://doi.org/10.1007/s13197-019-04092-y
Zia-ul-Haq M, Stanković MS, Rizwan K, de Feo V (2013) Grewia asiatica L., a food plant with multiple uses. Molecules 18:2663–2682. https://doi.org/10.3390/molecules18032663
Morton JF (1987) Phalsa. In: Fruits of Warm Climates. Julia F. Morton Publisher, 20534 SW 92 Ct., Miami, Florida, pp 276–277
Qamar M, Akhtar S, Ismail T, Sestili P, Tawab A, Ahmed N (2020) Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (phalsa). J Berr Res 10(1):115–131. https://doi.org/10.3233/JBR-190459
Khattab HA, El-Shitany NA, Abdallah IZ, Yousef FM, Alkreathy HM (2015) Antihyperglycemic potential of Grewia asiatica fruit extract against streptozotocin-induced hyperglycemia in rats: anti-inflammatory and antioxidant mechanisms. Oxidative Med Cell Longev 2015:549743. https://doi.org/10.1155/2015/549743
Sharma KV, Sisodia R (2009) Evaluation of the free radical scavenging activity and radioprotective efficacy of Grewia asiatica fruit. J Radiol Prot 29:429–443. https://doi.org/10.1088/0952-4746/29/3/007
Yadav AK (1999) Phalsa: a potential new small fruit for Georgia. In: Janik J (ed) Perspectives on new crops and new uses. ASHS Press, Alexandria, VA, USA, pp 348–352
Khan AS, Hussain A, Khan F (2006) Nutritional importance of micronutrients in some edible wild and unconventional fruits. J Chem Soc Pak 28:576–582
Gupta MK, Sharma PK, Ansari SH, Lagarkha R (2006) Pharmacognostical evaluation of Grewia asiatica fruits. Int J Plant Sci 1:249–251
Talpur MK, Talpur FN, Balouch A, Nizamani S, Surhio M, Shah MR, Bhanger MI, Afridi HI (2017) Analysis and characterization of anthocyanin from phalsa (Grewia asiatica). MOJ Food Proc Technol 5(3):299–305. https://doi.org/10.15406/mojfpt.2017.05.00127
Talpur MK, Talpur FN, Balouch A, Nizamani SM, Surhio MA, Shah MR, Bhanger MI, Afridi HI, Qambrani S (2021) Highly selective purification of Grewia asiatica anthocyanin based on macroporous resins. Pak J Analyt Environ Chem 22(1):44–52. https://doi.org/10.21743/pjaec/2021.06.06
AOAC (2019) Official methods of analysis of the association of official analytical chemists: official methods of analysis of AOAC International, 21st edn. AOAC, Washington DC
Abushita AA, Hebshi EA, Daood HG, Biacs PA (1997) Determination of antioxidant vitamins in tomatoes. Food Chem 60:207–212. https://doi.org/10.1016/S0308-8146(96)00321-4
Singleton VL, Rossi JJA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Denev P, Kratchanova M, Ciz M, Lojek A, Vasicek O, Nedelcheva P, Blazheva D, Toshkova R, Gardeva E, Yossifova L, Hyrsl P, Vojtek L (2014) Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem 157:37–44. https://doi.org/10.1016/j.foodchem.2014.02.022
Brand-Williams W, Cuvelier M, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT - Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Saeed A, Kausar S, Iqbal M (2018) Nutrient, mineral, antioxidant, and anthocyanin profiles of different cultivars of Syzygium cumini (Jamun) at different stages of fruit maturation. Pak J Bot 50(5):1791–1804
Ayaz FA, Kadioglu A, Bertoft E, Acar C, Turna I (2010) The effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos L. and V. myrillus L.) native to Turkey. NZJ Crop Hortic Sci 29:137–141. https://doi.org/10.1080/01140671.2001.9514171
Wu BH, Quilot B, Génard M, Kervella J, Li SH (2005) Changes in sugar and organic acid concentrations during fruit maturation in peaches, P. davidiana and hybrids as analyzed by principal component analysis. Sci Hortic 103:429–439. https://doi.org/10.1016/j.scienta.2004.08.003
Wu J, Xu Z, Zhang Y, Chai L, Yi H, Deng X (2014) An integrative analysis of the transcriptome and proteome of the pulp of a spontaneous late ripening sweet orange mutant and its wild type improves our understanding of fruit ripening in citrus. J Exp Bot 65:1651–1671. https://doi.org/10.1093/jxb/eru044
Glew RH, Ayaz FA, Sanz C, VanderJagt DJ, Huang HS, Chuang LT, Strand M (2003) Changes in sugar, organic acids and amino acids in medlar (Mespilus germanica L.) during fruit development and maturation. Food Chem 83:363–369. https://doi.org/10.1016/S0308-8146(03)00097-9
Mahmood T, Anwar F, Abbas M, Boyce MC, Saari N (2012) Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from Pakistan. Int J Mol Sci 13(2):1380–1392. https://doi.org/10.3390/ijms13021380
Ivancic T, Jakopic J, Veberic R, Vesel V, Hudina M (2022) Effect of ripening on the phenolic and sugar contents in the meso- and epicarp of Olive fruits (Olea europaea L.) cultivar ‘Leccino’. Agriculture 12(9):1347. https://doi.org/10.3390/agriculture12091347
Tao J, Wu M, Jiao X, Chen S, Jia D, Xu X, Huang C (2022) Dynamic changes of fruit physiological quality and sugar components during fruit growth and development of Actinidia eriantha. Horticulturae 8:529. https://doi.org/10.3390/horticulturae8060529
Samkumar A, Karppinen K, Dhakal B, Martinussen I, Jaakola L (2022) Insights into sugar metabolism during bilberry (Vaccinium myrtillus L.) fruit development. Physiol Plant 174(2):e13657. https://doi.org/10.1111/ppl.13657
Vallarino JG, de Abreu ELF, Soria C, Tong H, Pott DM, Willmitzer L, Fernie AR, Nikoloski Z, Osorio S (2018) Genetic diversity of strawberry germplasm using metabolomic biomarkers. Sci Rep 8:14386. https://doi.org/10.1038/s41598-018-32212-9
Xu SM, Brill E, Llewellyn DJ, Furbank RT, Ruan YL (2012) Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Mol Plant 5:430–441. https://doi.org/10.1093/mp/ssr090
Tognetti JA, Pontis HG, Martınez-Noël GMA (2013) Sucrose signaling in plants: a world yet to be explored. Plant Signal Behav 8:e23316. https://doi.org/10.4161/psb.23316
Lecourieux F, Kappel C, Lecourieux D, Serrano A, Torres E, Arce-Johnson P, Delrot S (2014) An update on sugar transport and signalling in grapevine. J Exp Bot 65:821–832. https://doi.org/10.1093/jxb/ert394
Zhao J, Li H, Xi W, An W, Niu L, Cao Y, Wang H, Wang Y, Yin Y (2015) Changes in sugars and organic acids in wolfberry (Lycium barbarum L.) fruit during development and maturation. Food Chem 173:718–724. https://doi.org/10.1016/j.foodchem.2014.10.082
Léchaudel M, Joas J, Caro Y, Génard M, Jannoyer M (2005) Leaf: fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. J Agric Food Chem 85:251–260. https://doi.org/10.1002/jsfa.1968
Díaz-Mula HM, Castillo S, Martínez-Romero D, Valero D, Zapata PJ, Guillén F, Serrano M (2009) Sensory, nutritive and functional properties of sweet cherry as affected by cultivar and ripening stage. Food Sci Technol Int 15:535–544. https://doi.org/10.1177/1082013209351868
Ercisli S, Orhan E (2008) Some physico-chemical characteristics of black mulberry (Morus nigra L.) genotypes from Northeast Anatolia region of Turkey. Sci Hortic 116:41–46. https://doi.org/10.1016/j.scienta.2007.10.021
Akhtar I, Rab A (2015) Effect of fruit ripening stages on strawberry (Fragaria x ananassa. Duch) fruit quality for fresh consumption. J Agric Res 53(3):413–424
Rahman MM, Moniruzzaman M, Ahmad MR, Sarker BC, Khurshid AM (2016) Maturity stages affect the postharvest quality and shelf-life of fruits of strawberry genotypes growing in subtropical regions. J Saudi Soc Agric Sci 15(1):28–37. https://doi.org/10.1016/j.jssas.2014.05.002
Batista-Silva W, Nascimento VL, Medeiros DB, Nunes-Nesi A, Ribeiro DM, Zsögön A, Araújo WL (2018) Modifications in organic acid profiles during fruit development and ripening: correlation or causation? Front Plant Sci 9:1689. https://doi.org/10.3389/fpls.2018.01689
Cherian S, Figueroa CR, Nair H (2014) Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus nonclimacteric fruit. J Exp Bot 65:4705–4722. https://doi.org/10.1093/jxb/eru280
Cosme Silva GM, Silva WB, Medeiros DB, Salvador AR, Cordeiro MHM, da Silva NM, Santana DB, Mizobutsi GB (2017) The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. palmer) fruit during storage. Food Chem 237:372–378. https://doi.org/10.1016/j.foodchem.2017.05.123
Agrumes F, Citrusfruecht G, Agrumi I, Agrios S, Monselise SP (2018) Citrus. In: Monselise SP (ed) Handbook of fruit set and development. CRC Press, Boca Raton, FL, pp 87–108
Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C (2013) What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot 64:1451–1469. https://doi.org/10.1093/jxb/ert035
Corrêa CV, Gouveia AM, Martins BN, Jorge LG, de BL Lanna N, Tavares AE, Mendonça VZ, Evangelista RM (2017) Influence of ripening stages on physicochemical characteristics of acerola fruits. Revis de Ciênc Agrár 40(4):808–813. https://doi.org/10.19084/RCA17116
Deng H, Xia H, Guo Y, Liu X, Lin L, Wang J, Xu K, Lv X, Hu R, Liang D (2022) Dynamic changes in ascorbic acid content during fruit development and ripening of Actinidia latifolia (an ascorbate-rich Fruit crop) and the associated molecular mechanisms. Int J Mol Sci 23:5808. https://doi.org/10.3390/ijms23105808
El-Beltagi HS, Ullah I, Sajid M, Basit A, Shehata WF, Shah ST, Alturki SM, Ullah A, Aziz I, Ali F (2022) Influence of maturity stages on postharvest physico-chemical properties of grapefruit (Citrus paradisi var. ‘Shamber Tarnab’) under different storage durations. Not Bot Horti Agrobot 50(1):12620. https://doi.org/10.15835/nbha50112620
El-Beltagi HS, Mohamedl HI, Safwatl G, Gamall M, Megahed BMH (2019a) Chemical composition and biological activity of Physalis peruviana L. Gesunde Pflanz 71:113–122. https://doi.org/10.1007/s10343-019-00456-8
El-Beltagi HS, Mohamed HI, Elmelegy AA, Eldesoky SE, Safwat G (2019b) Phytochemical screening, antimicrobial, antiaxidant, anticancer activities and nutritional values of Cactus (Opuntia ficus Indicia) pulp and peel. Fresenius Environ Bull 28:1534–1551
Emese J, Nagymat PF (2008) The stability of vitamin C in different beverages. Br Food J 110(3):296–309. https://doi.org/10.1108/00070700810858709
Cepeda JS, Bringas E, Balz M (1993) Ascorbic acid and quality losses of Valencia oranges stored on trees. Hortic Sci 28:581
Butt VS (1980) Direct oxidases and related enzymes. In: Stumpf PK, Conn EE (eds) The biochemistry of plants: a comprehensive treatise. Academic, New York, pp 81–123
Nogueira RJMC, Moraes JAPV, Burity HA, Silva Junior JF (2002) Efeito do estádio de maturação dos frutos nas características físico-químicas da acerola. Pesqui Agropecu Bras 37(4):463–470. https://doi.org/10.1590/S0100-204X2002000400006
Cormick G, Belizán JM (2019) Calcium intake and health. Nutrients 11(7):1606. https://doi.org/10.3390/nu11071606
Khan KY, Khan MA, Niamat R, Munir M, Fazal H, Mazari P, Seema N, Bashir T, Kanwal A, Ahmed SN (2011) Element content analysis of plants of genus Ficus using atomic absorption spectrometer. Afr J Pharm Pharmacol 5:317–321. https://doi.org/10.5897/AJPP10.339
Hussain A, Sajid M, Potter D, Rasheed H, Hassan M, Akhtar N, Ahmad B, Bokhari SAI (2023) Diversity in elemental content in selected Artemisia L. (Asteraceae) species from Gilgit-Baltistan region of Pakistan based on inductively coupled plasma atomic emission spectrophotometry (ICP-AES). Biol Trace Elem Res 201(8):4143–4155. https://doi.org/10.1007/s12011-022-03469-z
World Health Organization (2004) Vitamin and mineral requirements in human nutrition. 2nd ed. WHO, Geneva, Switzerland
Zheng W, You C, Du Z, Zhai H (2006) Dynamic changes in the calcium content of several apple cultivars during the growing season. Agric Sci China 5(12):933–937. https://doi.org/10.1016/s1671-2927(07)60007-8
Seyhan F, Ozay G, Saklar S, Ertaş E, Satir G, Alasalvar C (2007) Chemical changes of three native Turkish hazelnut varieties (Corylus avellana L.) during fruit development. Food Chem 105:590–596
Ram S, Sing RP (2018) Biochemical evaluation of mineral profile and ascorbic acid content in various varieties of ripened mango (Mangifera indica L.) fruits. Int J Curr Microbiol App Sci 7:2801–2805
Chiveu J, Naumann M, Kehlenbeck K, Pawelzik E (2019) Variation in fruit chemical and mineral composition of Kenyan guava (Psidium guajava L.): inferences from climatic conditions, and fruits morphological traits. J Appl Bot Food Qual 92:151–159
Fu XZ, Xie F, Cao L, Ling LL, Chun CP, Peng LZ (2019) Changes in mineral nutrition during fruit growth and development of “Seike” and “Newhall” navel orange as a guide for fertilization. Revist Brasil Fruticul Jabot 41(5):e–111. https://doi.org/10.1590/0100-2945201911
Kumari D, Prasad M, Ahmad F (2020) Variation among different growth stages on mineral nutrient content in guava fruits. Curr Appl Sci Technol 39(2):140–144
Rana SS, Pradhan RC, Mishra S (2018) Variation in properties of tender jackfruit during different stages of maturity. J Food Sci Technol 55(6):2122–2129. https://doi.org/10.1007/s13197-018-3127-9
Buwalda JG, Meekings JS (1990) Seasonal accumulation of mineral nutrients in leaves and fruit of Japanese pear (Pyrus serotina Rehd.). Sci Hortic 41:209–222. https://doi.org/10.1016/0304-4238(90)90004-X
Hocking B, Tyerman SD, Burton RA, Gilliham M (2016) Fruit calcium: transport and physiology. Front Plant Sci 7:569. https://doi.org/10.3389/fpls.2016.00569
Verdoucq L, Grondin A, Maurel C (2008) Structure-function analysis of plant aquaporin AtPIP2; 1 gating by divalent cations and protons. Biochem J 415:409–416. https://doi.org/10.1042/BJ20080275
Gilliham M, Dayod M, Hocking BJ, Xu B, Conn SJ, Kaiser BN, Leigh RA, Tyerman SD (2011) Calcium delivery and storage in plant leaves: exploring the link with water flow. J Exp Bot 62:2233–2250. https://doi.org/10.1093/jxb/err111
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress, and human health. Mol Asp Med 26(4–5):268–298. https://doi.org/10.1016/j.mam.2005.07.015
Zheng Y, Wang L, Dixon M (2004) Response to copper toxicity for three ornamental crops in solution culture. Hort Sci 39(5):1116–1120. https://doi.org/10.21273/HORTSCI.39.5.1116
Fernandes JC, Henriques FS (1991) Biochemical, physiological, and structural effects of excess copper in plants. Bot Rev 57:247–273. https://doi.org/10.1007/BF02858564
Malik AR, Mushtaq R, Kirmani SN, Bhat KM, Ganie MA, Wani SM, Bhat R, Soundri AS, Banday S, El-Serehy H (2023) Nutrient changes in berries of “Anab-e-Shahi” and “Perllete” varieties of grapes with advancing phenology in the growing season. Horticulturae 9:178. https://doi.org/10.3390/horticulturae9020178
Wessling-Resnick M (2014) Iron. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler RG (eds) Modern nutrition in health and disease, 11th edn. Lippincott Williams & Wilkins, Baltimore, MD, pp 176–188
Aggett PJ (2012) Iron. In: Erdman JW, Macdonald IA, Zeisel SH (eds) Present knowledge in nutrition, 10th edn. Wiley-Blackwell, Washington, DC, pp 506–520
Institute of Medicine (2001) Food and Nutrition Board. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: a report of the panel on micronutrients. National Academy Press, Washington, DC https://www.ncbi.nlm.nih.gov/books/NBK222317/
Laszlo JA (1991) Changes in endogenous and exogenous iron-reducing capability of soybean hull during development. Cereal Chem 68(1):21–24
Rude RK (2012) Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (eds) Modern nutrition in health and disease, 11th edn. Lippincott Williams & Wilkins, Baltimore, Mass, pp 159–175
Moshfegh AJ, Goldman JD, Ahuja JK, Rhodes DG, Lacomb RP (2009) What we eat in America, NHANES 2005–2006, usual nutrient intakes from food and water compared to 1997 dietary reference intakes for vitamin D, calcium, phosphorus, and magnesium. http://www.ars.usda.gov/ba/bhnrc/fsrg
Tarleton EK (2018) Factors influencing magnesium consumption among adults in the United States. Nutr Rev 76(7):526–538. https://doi.org/10.1093/nutrit/nuy002
Salomão LCC, Siqueira D, Pereira MEC (2006) Acúmulo de macro e micronutrientes nas folhas e caules do ramo produtivo da lichieira “Bengal” durante um ano. Ciênc Agrotecnol 30(1):914. https://doi.org/10.1590/S1413-70542006000100001
Giancarlo R, Damiano Z, Maria A, Antonio G, Torrisi BF, Leonardi A, Quiñones A, Intrigliolo F, Tagliavini M (2012) Assessing nutrient uptake by field grown orange trees. Eur J Agron 41:73–80. https://doi.org/10.1016/j.eja.2012.03.011
Arena ME, Povilonis IS, Borroni V, Pérez E, Pellegrino N, Cacciatore C, Radice S (2023) Changes in carbohydrates, organic acids, and minerals at different development stages of Hexachlamys edulis fruit, a wild South American species with horticultural potential. Horticulturae 9:314. https://doi.org/10.3390/horticulturae9030314
Zhang B, Cakmak I, Feng J, Yu C, Chen X, Xie D, Wu L, Song Z, Cao J, He Y (2020) Magnesium deficiency reduced the yield and seed germination in wax gourd by affecting the carbohydrate translocation. Front Plant Sci 11:797. https://doi.org/10.3389/fpls.2020.00797
Wahid F, Fahad S, Danish S, Adnan M, Yue Z, Saud S, Siddiqui MH, Brtnicky M, Hammerschmiedt T, Datta R (2020) Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 10(8):334. https://doi.org/10.3390/agriculture10080334
Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR (2014) Modern nutrition in health and disease. Lippincott Williams and Wilkins, Philadelphia, PA, USA 11th edition
Calvo MS, Uribarri J (2013) Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr 98(1):6–15. https://doi.org/10.3945/ajcn.112.053934
Moshfegh AJ, Kovalchik AF, Clemens JC (2016) Phosphorus intake of the US population: what we eat in America. In: NHANES 2011–2012. Food surveys research group dietary data brief no. 15, 2016. USDA, Research, Education & Economics Information System, Beltsvile, MD
Marschner H (2012) Mineral nutrition of higher plants, 3rd edn. Academic Press, London, UK
White PJ, Karley AJ (2010) Potassium cell biology of metals and nutrients. Springer, Berlin, pp 199–224
Oosterhuis D, Loka D, Kawakami E, Pettigrew W (2014) The physiology of potassium in crop production. Adv Agron 126:203–234. https://doi.org/10.1016/B978-0-12-800132-5.00003-1
WHO (2012a) Guideline: sodium Intake for adults and children. World Health Organization, Geneva
Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP (2013) Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 346:f1378–f1378. https://doi.org/10.1136/bmj.f1378
Zhang Z, Yang F, Tian X (2009) Coronatine-induced lateral formation in cotton (Gossypium hirsutum) seedlings under potassium-sufficient and –deficient conditions in relation to auxin. J Plant Nutr Soil Sci 172:435–444. https://doi.org/10.1002/jpln.200800116
Kronzucker HJ, Coskun D, Schulze LM, Wong JR, Britto DT (2013) Sodium as nutrient and toxicant. Plant Soil 369:1–23. https://doi.org/10.1007/s11104-013-1801-2
WHO (2012b) Guideline: potassium intake for adults and children. World Health Organization, Geneva
Prueitt RL, Li W, Chang YC, Boffetta P, Goodman JE (2020) Systematic review of the potential respiratory carcinogenicity of metallic nickel in humans. Crit Rev Toxicol 50(7):605–639. https://doi.org/10.1080/10408444.2020.1803792
Nielsen FH (1996) Other trace elements. In: Ziegler EE, Filer LJ Jr (eds) Present knowledge in nutrition, 7th edn. Washington (DC), International Life Sciences Institute, pp 353–377
Trumbo P, Yates AA, Schlicker S, Poos M (2001) Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101(3):294–301. https://doi.org/10.1016/S0002-8223(01)00078-5
Qadir R, Anwar F, Bashir K, Tahir MH, Alhumade H, Mehmood T (2022) Variation in nutritional and antioxidant attributes of Moringa oleifera L. leaves at different maturity stages. Front Ener Res 10:888355. https://doi.org/10.3389/fenrg.2022.888355
Meindl GA, Bain DJ, Ashman T-L (2014) Nickel accumulation in leaves, floral organs and rewards varies by serpentine soil affinity. AoB Plants 6:plus036. https://doi.org/10.1093/aobpla/plu036
Jansen J, Karges W, Rink L (2009) Zinc and diabetes—clinical links and molecular mechanisms. J Nutr Biochem 20:399–417. https://doi.org/10.1016/j.jnutbio.2009.01.009
Siddiqi KS, ur Rahman A, Tajuddin HA (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanosc Res Lett 13:141. https://doi.org/10.1186/s11671-018-2532-3
Lang A, Düring H (1991) Partitioning control by water potential gradient; evidence for compartmentation breakdown in grape berries. J Exp Bot 42:1117–1122. https://doi.org/10.1093/jxb/42.9.1117
Bondada BR, Matthews MA, Shackel KA (2005) Functional xylem in the post-veraison grape berry. J Exp Bot 56(421):2949–2957. https://doi.org/10.1093/jxb/eri291
Sukrasno N, Yeoman MM (1993) Phenylpropanoid metabolism during growth and development of Capsicum frutescens fruits. Phytochemistry 32:839–844. https://doi.org/10.1016/0031-9422(93)85217-F
Kondo S, Tsuda K, Muto N, Ueda JE (2002) Antioxidative activity of apple skin or flesh extracts associated with fruit development on selected apple cultivars. Sci Hortic 96:177–185. https://doi.org/10.1016/S0304-4238(02)00127-9
Tlili N, Mejri H, Yahia Y, Saadaoui E, Rejeb S, Khaldi A (2014) Phytochemicals and antioxidant activities of Rhus tripartitum (urcia) fruits depending on locality and different stages of maturity. Food Chem 160:98–103. https://doi.org/10.1016/j.foodchem.2014.03.030
Wang B, Huang Q, Venkitasamy C, Chai H, Gao H, Cheng N, Cao W, Lv X, Pan Z (2016) Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT - Food Sci Technol 66:56–62. https://doi.org/10.1016/j.lwt.2015.10.005
Wang Y, Qi D, Wang S, Cao X, Ye Y, Suo Y (2018) Comparison of phenols content and antioxidant activity of fruits from different maturity stages of Ribes stenocarpum Maxim. Molecules 23(12):3148. https://doi.org/10.3390/molecules23123148
Wojdyło A, Oszmiański J (2020) Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants 9:567. https://doi.org/10.3390/antiox9070567
Zhang H, Pu J, Tang Y, Wang M, Tian K, Wang Y, Luo X, Deng Q (2022) Changes in phenolic compounds and antioxidant activity during development of ‘Qiangcuili’ and ‘Cuihongli’ fruit. Foods 11:3198. https://doi.org/10.3390/foods11203198
Nadeem M, Zeb A (2018) Impact of maturity on phenolic composition and antioxidant activity of medicinally important leaves of Ficus carica L. Physiol Mol Biol Plants 24(5):881–887. https://doi.org/10.1007/s12298-018-0550-3
Hou J, Liang L, Su M, Yang T, Mao X, Wang Y (2021) Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem 360:129980. https://doi.org/10.1016/j.foodchem.2021.12998
Dong X, Hu Y, Li Y, Zhou ZJSH (2019) The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: a negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Sci Hortic 243:281–289. https://doi.org/10.1016/j.scienta.2018.08.036
Undurraga PL, Olaeta JA, Retamales JB, Escobar J, Toso AM (2009) Effect of maturity and storage temperature on the development of peteca in lemons (Citrus limon (L.). Burm. F.) cv. Eureka. Sci Hortic 122(1):56–61. https://doi.org/10.1016/j.scienta.2009.03.026
He JG, Cheng YD, Guan JF, Ge WY, Zhao Z (2017) Changes of chlorogenic acid content and its synthesis−associated genes expression in Xuehua pear fruit during development. J Integr Agric 16:471–477
Hoxha L, Kongoli R, Dervishi J (2022) Influence of maturity stage on polyphenolic content and antioxidant activity of Fig (Ficus carica L.) fruit in native Albanian varieties. Chem Proc 10:–49. https://doi.org/10.3390/IOCAG2022-12199
Alarcao-e-Silva MLCMM, Leitao AEB, Azinheira HG, Leitao MCA (2001) The arbutus berry: studies on its color and chemical characteristics at two mature stages. J Food Compos Anal 14:27–35. https://doi.org/10.1006/jfca.2000.0962
Zoratti L, Sarala M, Carvalho E, Karppinen K, Martens S, Giongo L, Häggman H, Jaakola L (2014) Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Biol 14:377. https://doi.org/10.1186/s12870-014-0377-1
Acosta-Montoya Ó, Vaillant F, Cozzano S, Mertz C, Pérez AM, Castro MV (2010) Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem 119:1497–1501. https://doi.org/10.1016/j.foodchem.2009.09.032
Li J, Shi C, Shen D, Han T, Wu W, Lyu L, Li W (2022) Composition and antioxidant activity of anthocyanins and non-anthocyanin flavonoids in Blackberry from different growth stages. Foods 11:2902. https://doi.org/10.3390/foods11182902
Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89:569–575. https://doi.org/10.1016/j.foodchem.2004.03.013
Saikia S, Mahnot NK, Mahanta CL (2016) Phytochemical content and antioxidant activities of thirteen fruits of Assam. India. Food Biosci 13:15–20. https://doi.org/10.1016/j.fbio.2015.11.003
Riaz M, Zia-Ul-Haq M, Saad B (2016) Biosynthesis and stability of anthocyanins. In: Anthocyanins and human health: biomolecular and therapeutic aspects. Springer, Cham, Switzerland, pp 71–86
Eker ME, Aaby K, Budic-Leto I, Rimac BS, El SN, Karakaya S, Simsek S, Manach C, Wiczkowski W, de Pascual-Teresa S (2020) A review of factors affecting anthocyanin bioavailability: possible implications for the inter-individual variability. Foods 9(1):2. https://doi.org/10.3390/foods9010002
Martín J, Kuskoski EM, Navas MJ, Asuero AG (2017) Antioxidant capacity of anthocyanin pigments. Flavon-From Biosyn Hum Health 3:205–255. https://doi.org/10.5772/67718
Zhao Q, Duan CQ, Wang J (2010) Anthocyanins profile of grape berries of Vitis amurensis, its hybrids and their wines. Int J Mol Sci 11(5):2212–2218. https://doi.org/10.3390/ijms11052212
Singh MC, Kelso C, Price WE, Probst Y (2020) Validated liquid chromatography separation methods for identification and quantification of anthocyanins in fruits and vegetables: a systematic review. Food Res Int 138:109754. https://doi.org/10.1016/j.foodres.2020.109754
Agarwal S, Mishra K (1979) New flavonoids from the Grewia asiatica. J Indian Chem Soc 56(6):649
Khurdiya DS, Anand JC (1981) Anthocyanin derivatives from the fruits of Grewia asiatica. J Food Sci Technol 18:112–114
Mabika ABM, Nagakegni-Limbili AC, Agnaniet H, Nyegue MA, Zhao JMQ, Thiery V, Ouamba JM (2017) Analytical characterizations of anthocyanins of the hydro-alcoholic extract fruits of Grewia Coriacea Mast. Pharm et Méd Trad Afrs 18:23–30
Rajavel T, Mohankumar R, Archunan G, Ruckmani K, Devi KP (2017) Beta sitosterol and daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep 7:1–15. https://doi.org/10.1038/s41598-017-03511-4
Fan-Chiang HJ, Wrolstad RE (2005) Anthocyanin pigment composition of blackberries. J Food Sci 70:C198–C202. https://doi.org/10.1111/j.1365-2621.2005.tb07125.x
Shim Y, Yoon W, Kim D, Watanabe M, Park H, Jang HW, Lee J, Ha J (2015) The simple determination method for anthocyanidin aglycones in fruits using ultra-high-performance liquid chromatography. J Chromatogr Sci 53(10):1646–1653. https://doi.org/10.1093/chromsci/bmv064
Hutabarat RP, Xiao YD, Wu H, Wang J, Li DJ, Huang WY (2019) Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J Food Qual 2019:1–10. https://doi.org/10.1155/2019/6806790
Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L (2010) Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol 127(1):7–10. https://doi.org/10.1016/j.jep.2009.09.059
Acknowledgements
The authors extend their gratefulness to the anonymous reviewers for their appreciated remarks/critics to improve the quality of the manuscript.
Funding
This work received funds from the Pakistan Science Foundation (PSF), Islamabad, Pakistan, under Grant no. PSF/NSLP/P-PCSIR(195) awarded to the first author (Dr. Asma Saeed).
Author information
Authors and Affiliations
Contributions
A.S. conceptualized and designed the research, collected fruit sample, performed formal analysis, analyzed the data, performed statistical analysis, acquired funds, administered the project, and drafted the initial version of the manuscript. S.K. performed lab experimentation, analyzed the data and drafted the initial version of the manuscript. A.H. prepared and edited the final version of the manuscript, and reviewed the manuscript for grammar and language errors. N.J.S. performed lab experimentation and analyzed the data. S.H.I.A., Q.S., and A.A.N. reviewed the manuscript and validated the data. All the authors reviewed this final version of the manuscript and approved its submission.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saeed, A., Kauser, S., Hussain, A. et al. Tracking the Variations in Trace Elements, Some Nutrients, Phenolics, and Anthocyanins in Grewia asiatica L. (Phalsa) at Different Fruit Development Stages. Biol Trace Elem Res 202, 1784–1801 (2024). https://doi.org/10.1007/s12011-023-03763-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03763-4