Abstract
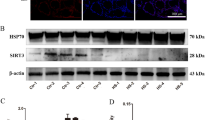
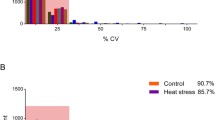
Heat stress threatens severely cardiac function by caused myocardial injury in poultry. Our previous study has showed that manganese (Mn) has a beneficial effect on heat-stress resistance of broiler. Therefore, we tried to confirm the alleviation mechanism through proteomic analysis after heat stress exposure to primary broiler myocardial cells pretreated with Mn. The experiment was divided into four groups: CON group (37 °C, cells without any treatment), HS group (43 °C, cells treatment with heat stress for 4 h), HS+MnCl2 group (cells treated with 20 μM MnCl2 before heat stress), and HS+Mn-AA group (cells treated with 20 μM Mn compound amino acid complex before heat stress). Proteome analysis using DIA identified 300 differentially expressed proteins (DEPs) between CON group and HS group; 93 and 121 DEPs were identified in inorganic manganese treatment group and organic manganese treatment group, respectively; in addition, there were 53 DEPs identified between inorganic and organic manganese group. Gene Ontology (GO) analysis showed that DEPs were mainly involved in binding, catalytic activity, response to stimulus, and metabolic process. DEPs of manganese pretreatment involved in a variety of biological regulatory pathways, and significantly influenced protein processing and repair in endoplasmic reticulum, apoptosis, and DNA replication and repair. These all seem to imply that manganese may help to resist cell damage induced by heat stress by regulating key node proteins. These findings contribute to a better understanding of the effects of manganese on overall protein changes during heat-stress and the possible mechanisms, as well as how to better use manganese to protect heart function in high temperature.






Similar content being viewed by others
References
Jahejo AR, Rajput N, Rajput NM, Leghari IH, Kaleri RR, Mangi RA, Sheikh MK, Pirzado MZ (2016) Effects of heat stress on the performance of Hubbard broiler chicken. Cells 2:1–5
Zaboli GR, Rahimi S, Shariatmadari F, Torshizi MAK, Baghbanzadeh A, Mehri M (2017) Thermal manipulation during pre and post-hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult Sci 96(2):478–485. https://doi.org/10.3382/ps/pew344
Kamel NN, Ahmed AMH, Mehaisen GMK, Mashaly MM, Abass AO (2017) Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int J Biometeorol 61(9):1637–1645. https://doi.org/10.1007/s00484-017-1342-0
St-Pierre NR, Cobanov B, Schnitkey G (2003) Economic losses from heat stress by US livestock industries. J Dairy Sci 86:E52–E77. https://doi.org/10.3168/jds.S0022-0302(03)74040-5
Chang CP, Hsu YC, Lin MT (2003) Magnolol protects against cerebral ischaemic injury of rat heatstroke. Clin Exp Pharmacol P 30(5-6):387–392. https://doi.org/10.1046/j.1440-1681.2003.03847.x
Wu D, Xu J, Song E, Tang S, Zhang XH, Kemper N, Hartung J, Bao E (2015) Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperon 20(4):687–696. https://doi.org/10.1007/s12192-015-0596-x
Xu Y, Jie Z, Lin L, Bo Y, Chen BX, Bao ED, Zhang XH (2023) Hsp90 protected chicken primary myocardial cells from heat-stress injury by inhibiting oxidative stress and calcium overload in mitochondria. Biochem Pharmacol 209:115434
Yang L, Tan GY, Fu YQ, Feng JH, Zhang MH (2010) Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Phys C 151(2):204–208. https://doi.org/10.1016/j.cbpc.2009.10.010
Slimen IB, Najar T, Ghram A, Dabbebi H, Mrad MB, Abdrabbah M (2014) Reactive oxygen species, heart stress and oxidative-induced mitoehondrial damage. A review Int J Hyperther 30(7):513–523. https://doi.org/10.3109/02656736.2014.971446
Gu XH, Hao Y, Wang XL (2012) Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2 intestinal oxidative stress. Poultry Sci 91(4):790–799. https://doi.org/10.3382/ps.2011-01628
Víctor MV, Milagros R, Mónica DLF (2003) Regulation of macrophage function by the antioxidant N-acetylcysteine in mouse-oxidative stress by endotoxin. Int Immunopharmacol 3(1):97–106. https://doi.org/10.1016/s1567-5769(02)00232-1
Zeng T, Li JJ, Wang DQ, Li GQ, Wang GL, Lu LZ (2014) Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperon 19(6):895–901. https://doi.org/10.1007/s12192-014-0514-7
Zhu YE, Lu L, Liao XD, Li WX, Zhang LY, Ji C, Lin X, Liu HC, Jack O, Luo XG (2017) Maternal dietary manganese protects chick embryos against maternal heat stress via epigenetic-activated antioxidant and antiapoptotic abilities. Oncotarget 8(52):89665–89680. https://doi.org/10.18632/oncotarget.20804
Li SF, Lu L, Liao XD, Gao TQ, Wang F, Zhang LY, Xi L, Liu SB, Luo XG (2016) Manganese elevates manganese superoxide dismutase protein level through protein kinase C and protein tyrosine kinase. Biometals 29(2):265–274. https://doi.org/10.1007/s10534-016-9913-9
Qin SZ, Huang L, Lu L, Zhang LY, Guo YL, Xi L, Liao XD, Luo XG (2023) Manganese alleviates heat stress of primary cultured chick embryonic myocardial cells via enhancing manganese superoxide dismutase expression and attenuating heat shock response. J Therm Biol 112:103440
Liao XD, Zhu YW, Lu L, Li WX, Zhang LY, Ji C, Lin X, Luo XG (2019) Maternal manganese activates anti-apoptotic-related gene expressions via miR-1551 and miR-34c in embryonic hearts from maternal heat stress (Gallus gallus). J Therm Biol 84:190–199. https://doi.org/10.1016/j.jtherbio.2019.07.014
Monti C, Zilocchi M, Colugnat I, Alberio T (2019) Proteomics turns functional. J Proteomics 198:36–44. https://doi.org/10.1016/j.jprot.2018.12.012
Allison D (2015) DIA mass spectrometry. Nat Methods 12(1):35–35. https://doi.org/10.1038/nmeth.3234
Vidova V, Spacil Z (2017) A review on mass spectrometry-based quantitative proteomics: targeted and data independent acquisition. Anal Chim Acta 964:7–23. https://doi.org/10.1016/j.aca.2017.01.059
Floriou-Servou A, Lukas VZ, Luzia S, Oliver S, Mattia P, Anahita R, Alessio C, Beat T, Johannes B (2018) Distinct proteomic, transcriptomic and epigenetic stress-responses in dorsal and ventral hippocampus. Biol Psychiat 84(7):531–541. https://doi.org/10.1016/j.biopsych.2018.02.003
Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M (2015) Manganese is essential for neuronal health. Annu Rev Nutr 35:71–108. https://doi.org/10.1146/annurev-nutr-071714-034419
Gunter EJM Gavin CE, Aschner M Thomas EG (2013) Manganese neurotoxicity and the role of reactive oxygen species. Free Radical Bio Med 62:65-75. https://doi.org/10.1016/j.freeradbiomed.2013.01.032.
Lopez V, Heijden EVD, Villar M, Michel A, Alberdi P, Gortázar C, Rutten V, Fuente JDL (2018) Comparative proteomics identified immune response proteins involved in response to vaccination with heat-inactivated Mycobacterium bovis and mycobacterial challenge in cattle. Vet Immunol Immunop 206:54–64. https://doi.org/10.1016/j.vetimm.2018.10.013
Matsuki SG, Iuchi Y, Ikeda Y, Sasagawa I, Tomita Y, Fujii J (2003) Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Bioph Res Co 312(3):843–849. https://doi.org/10.1016/j.bbrc.2003.10.191
Saelao P, Wang Y, Chanthavixay G, Yu V, Gallardo RA, Dekkers JM (2018) Integrated proteomic and transcriptomic analysis of differential expression of chicken lung tissue in response to NDV infection during heat stress. Genes-Basel 9(12):579. https://doi.org/10.3390/genes9120579
Ari M,and Hrusti O (1975) Exposure to airborne manganese and arterial blood pressure. Environ Res 10(2):314-318. https://doi.org/10.1016/0013-9351(75)90092-4.
Meares GP, Zmijewska AA, Jope RS (2004) Heat shock protein-90 dampens and directs signaling stimulated by insulin-like growth factor-1 and insulin. FEBS Lett 574(1-3):181–186. https://doi.org/10.1016/j.febslet.2004.08.026
Langer T, Neupert W (1991) Heat shock proteins hsp60 and hsp70: their roles in folding, assembly and membrane translocation of proteins. Curr Top Microbiol 167:3–30. https://doi.org/10.1007/978-3-642-75875-1_1
Fang X, Bogomolovas J, Trexler C, Chen J (2019) The BAG3-dependent and -independent roles of cardiac small heat shock proteins. JCI Insight 4(4):e126464. https://doi.org/10.1172/jci.insight.126464
Carra S, Simon A, Arrigo PA, Benesch JL, Benjamin IJ (2017) The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperon 22(4):601–611. https://doi.org/10.1007/s12192-017-0787-8
Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J (2017) The chaperone activity and substrate spectrum of human small heat shock proteins. J Biol Chem 292(2):672–684. https://doi.org/10.1074/jbc.M116.760413
Depre C, Hase M, Gaussin V, Zajac A, Wang L, Hittinger L, Ghaleh B, Yu XZ, Kudej RK, Wagner T, Sadoshima J, Vatner SF (2002) H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ Res 91(11):1007–1014. https://doi.org/10.1161/01.RES.0000044380.54893.4B
Rauch JN, Tse E, Freilich R, Mok SA, Makley LN, Southworth DR, Gestwicki JE (2017) BAG3 is a modular, scaffolding protein that physically links heat shock protein 70 (Hsp70) to the small heat shock proteins. J Mol Cell Biol 429(1):128–141. https://doi.org/10.1016/j.jmb.2016.11.013
Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC (2003) Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol 82(10):523–530. https://doi.org/10.1078/0171-9335-00337
Carra S, Seguin SJ, Landry J (2008) HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy 4(2):237–239. https://doi.org/10.4161/auto.5407
Li FZ, Xiao H, Hu ZP, Zhou FF, Yang BB (2018) Exploring the multifaceted roles of heat shock protein B8 (HSPB8) in diseases. Eur J Cell Biol 97(3):216–229. https://doi.org/10.1016/j.ejcb.2018.03.003
Guilbert SM, Lambert H, Rodrigue MA, Fuchs M, Landry J, Lavoie JN (2018) HSPB8 and BAG3 cooperate to promote spatial sequestration of ubiquitinated proteins and coordinate the cellular adaptive response to proteasome insufficiency. Faseb J 32(7):3518–3535. https://doi.org/10.1096/fj.201700558RR
Mehlen P, Mehlen A, Godet J, Arrigo AP (1997) Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 272(50):31657–31665. https://doi.org/10.1074/jbc.272.50.31657
Doong H, Vrailas A, Kohn EC (2002) What’s in the ‘BAG’?–a functional domain analysis of the BAG-family proteins. Cancer Lett 188(1-2):25–32. https://doi.org/10.1016/s0304-3835(02)00456-1
Festa M, Valle LD, Khalili K, Franco R, Scognamiglio G, Graziano V, Laurenzi VD, Turco MC, Rosati A (2011) BAG3 protein is overexpressed in human glioblastoma and is a potential target for its therapy. Am J Surg Pathol 178(6):2504–2512. https://doi.org/10.1016/j.ajpath.2011.02.002
Rosati A, Graziano V, Laurenzi VD, Pascale M, Turco MC (2011) BAG3: a multifaceted protein that regulates major cell pathway. Cell Death Dis 2(4):e141. https://doi.org/10.1038/cddis.2011.24
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397(6718):441–446. https://doi.org/10.1038/17135
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410(6828):549–554. https://doi.org/10.1038/35069004
Norberg E, Orrenius S, Zhivotovsky B (2010) Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF). Biochem Bioph Res Co 396(1):95–100. https://doi.org/10.1016/j.bbrc.2010.02.163
Bonora M, Pedro JMB, Kroemer G, Galluzzi L, Pinton P (2014) Novel insights into the mitochondrial permeability transition. Cell Cycle. 13(17):2666–2670. https://doi.org/10.4161/15384101.2014.949082
Qin SZ, Wang R, Tang DF, Qin SJ, Guo YL, Shi ZG (2022) Manganese mitigates heat stress-induced apoptosis by alleviating endoplasmic reticulum stress and activating the NRF2/SOD2 pathway in primary chick embryonic myocardial cells. Biol Trace Elem Res 200:2312–2320. https://doi.org/10.1007/s12011-021-02810-2
Wang R, Shi ZG, Li JL, Tang DF, Qin SZ, Guo YL (2022) Protective effect of manganese on apoptosis and mitochondrial function of heat-stressed primary chick embryonic myocardial cells. Biol Trace Elem Res 200:4419–4429. https://doi.org/10.1007/s12011-021-03016-2
Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C (2003) Characterization of the signal that directs Bcl-x[sub L], but not Bcl-2, to the mitochondrial outer membrane. J Mol Cell Biol 160(1):53–64. https://doi.org/10.1083/jcb.200210084
Iftode C, Daniely Y, Borowiec JA (1999) Replication protein A (RPA): the eukaryotic SSB. Crit Rev Biochem Mol 34(3):141–180. https://doi.org/10.1080/10409239991209255
Burns JL, Guzder SN, Sung P, Prakash S, Prakash L (1996) An affinity of human replication protein A for ultraviolet-damaged DNA. J Biol Inorg Chem 271(20):11607–11610. https://doi.org/10.1074/jbc.271.20.11607
Costa RMA, Chiganças V, Galhardo RDS, Carvalho H, Menck CFM (2003) The eukaryotic nucleotide excision repair pathway. Biochimie 85(11):1083–1099. https://doi.org/10.1016/j.biochi.2003.10.017
Funding
This research was funded by the National Natural Science Foundation of China (No. 31960668) and Discipline Team Project of Gansu Agricultural University (NO: GAU-XKTD-2022-24).
Author information
Authors and Affiliations
Contributions
Shizhen Qin: conceptualization, methodology, writing—original draft preparation; Rui Wang: conceptualization, methodology, software; Jinlu Li: contributed materials, data curation, methodology; Defu Tang: methodology, software; Zhaoguo Shi: project administration, methodology.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, S., Wang, R., Li, J. et al. Quantitative Proteomics Reveals Manganese Alleviates Heat Stress of Broiler Myocardial Cells via Regulating Nucleic Acid Metabolism. Biol Trace Elem Res 202, 1187–1202 (2024). https://doi.org/10.1007/s12011-023-03731-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03731-y