Abstract
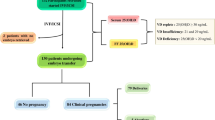
To explore the association between serum-related indicators (levels of inflammatory cytokines and essential trace elements) and miscarriage risk among infertile women undergoing assisted reproductive techniques (ART) on the 14th day after embryo transfer, and to develop and establish a multivariable algorithm model that might predict pregnancy outcome. According to a nested case–control study design, a total of 100 miscarriage cases and 100 live birth controls were included in this study, and women in both groups were infertile and have underwent in vitro fertilization (IVF). Pregnancy tests were performed and serum levels of five essential trace elements (vanadium (V), copper (Cu), zinc (Zn), selenium (Se) and molybdenum (Mo)) and five inflammatory cytokines (interleukin–1β (IL–1β), IL–6, IL–8, IL–10 and tumor necrosis factor–α (TNF–α)) of the participants were measured on the 14th day after embryo transfer. The serum levels of five inflammatory cytokines were determined by multiple magnetic bead enzyme immunity analyzer; and the serum concentrations of five elements were determined simultaneously by inductively coupled plasma‒mass spectrometry (ICP ‒ MS). The logistic regression was used to evaluate the relationship between these serum indices and miscarriage risk among women undergoing ART, and a predictive model of pregnancy outcome based on these indices was established. The levels of IL–10, IL–1β and TNF–α of infertile women in the live birth group were significantly higher than those in the miscarriage group (p = 0.009, p < 0.001, p = 0.006), and the levels of V, Cu, Zn and Se of infertile women in the live birth group were also significantly higher than those in the miscarriage group (all p < 0.001). Through logistic regression analyses, we found that serum levels of IL–1β, TNF–α, V, Cu, Zn and Se were significantly and negatively associated with miscarriage risk. Different combination prediction models were generated according to the results of logistic regression analyses, and the combination of IL–1β, Cu and Zn had the best prediction performance. The area under the curve (AUC) was 0.776, the sensitivity of the model was 60% and the specificity was 84%. In conclusion, the serum-related indicators of women undergoing ART on the 14th day after embryo transfer, including the inflammatory cytokines such as IL–1β and TNF–α and the essential trace metal elements such as V, Cu, Zn and Se, were negatively correlated with miscarriage risk. A multivariate algorithm model to predict pregnancy outcome among women undergoing ART was established, which showed that IL–1β, Cu and Zn might synergistically predict pregnancy outcome.



Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Piccinni MP (2002) T-cell cytokines in pregnancy. Am J Reprod Immunol 47(5):289–294.https://doi.org/10.1034/j.1600-0897.2002.01104.x
Schafer-Somi S (2003) Cytokines during early pregnancy of mammals: a review. Anim Reprod Sci 75(1–2):73–94.https://doi.org/10.1016/s0378-4320(02)00222-1
Seshagiri PB, Vani V, Madhulika P (2016) Cytokines and blastocyst hatching. Am J Reprod Immunol 75(3):208–217.https://doi.org/10.1111/aji.12464
Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP (2017) Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA 114(32):E6566–E6575.https://doi.org/10.1073/pnas.1701129114
Granot I, Gnainsky Y, Dekel N (2012) Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 144(6):661–668.https://doi.org/10.1530/REP-12-0217
Mor G, Cardenas I, Abrahams V, Guller S (2011) Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci 1221:80–87. https://doi.org/10.1111/j.1749-6632.2010.05938.x
Van Sinderen M, Menkhorst E, Winship A, Cuman C, Dimitriadis E (2013) Preimplantation human blastocyst-endometrial interactions: the role of inflammatory mediators. Am J Reprod Immunol 69(5):427–440.https://doi.org/10.1111/aji.12038
Kaislasuo J, Simpson S, Petersen JF, Peng G, Aldo P, Lokkegaard E, Paidas M, Pal L, Guller S, Mor G (2020) IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol 83(1):e13195.https://doi.org/10.1111/aji.13195
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12(9):1065–1074.https://doi.org/10.1038/nm1452
Kalkunte S, Nevers T, Norris WE, Sharma S (2011) Vascular IL-10: a protective role in preeclampsia. J Reprod Immunol 88(2):165–169.https://doi.org/10.1016/j.jri.2011.01.009
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med 26(4–5):235–244.https://doi.org/10.1016/j.mam.2005.07.013
Hostetler CE, Kincaid RL, Mirando MA (2003) The role of essential trace elements in embryonic and fetal development in livestock. Vet J 166(2):125–139.https://doi.org/10.1016/s1090-0233(02)00310-6
Mistry HD, Broughton PF, Redman CW, Poston L (2012) Selenium in reproductive health. Am J Obstet Gynecol 206(1):21–30.https://doi.org/10.1016/j.ajog.2011.07.034
Roychoudhury S, Nath S, Massanyi P, Stawarz R, Kacaniova M, Kolesarova A (2016) Copper-induced changes in reproductive functions: in vivo and in vitro effects. Physiol Res 65(1):11–22.https://doi.org/10.33549/physiolres.933063
Vickram S, Rohini K, Srinivasan S, Nancy VD, Archana K, Anbarasu K, Jeyanthi P, Thanigaivel S, Gulothungan G, Rajendiran N, Srikumar PS (2021) Role of zinc (Zn) in human reproduction: a journey from initial spermatogenesis to childbirth. Int J Mol Sci 22(4):2188. https://doi.org/10.3390/ijms22042188
Mehri A (2020) Trace elements in human nutrition (II) - an update. Int J Prev Med 11:2. https://doi.org/10.4103/2Fijpvm.IJPVM_48_19
Prohaska JR (2014) Impact of copper deficiency in humans. Ann N Y Acad Sci 1314:1–5.https://doi.org/10.1111/nyas.12354
Franklin RB, Costello LC (2007) Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys 463(2):211–217.https://doi.org/10.1016/j.abb.2007.02.033
Zalewski PD, Forbes IJ, Giannakis C (1991) Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem Int 24(6):1093–1101
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci 39(3):112–120.https://doi.org/10.1016/j.tibs.2013.12.007
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590.https://doi.org/10.1126/science.179.4073.588
Jahan TN, Anwar S, Kabir T, Hosen MJ (2022) Lead and lead-arsenic combined exposure induces mortality and developmental impairments in zebrafish embryos: a study using wild-caught zebrafish from Bangladesh. Drug Chem Toxicol 45(6):2833–2842.https://doi.org/10.1080/01480545.2021.1996594
Omeljaniuk WJ, Socha K, Soroczynska J, Charkiewicz AE, Laudanski T, Kulikowski M, Kobylec E, Borawska MH (2018) Cadmium and lead in women who miscarried. Clin Lab 64(1):59–67.https://doi.org/10.7754/Clin.Lab.2017.170611
Yıldırım E, Derici MK, Demir E, Apaydın H, Koçak Ö, Kan Ö, Görkem Ü (2019) Is the concentration of cadmium, lead, mercury, and selenium related to preterm birth? Biol Trace Elem Res 191(2):306–312.https://doi.org/10.1007/s12011-018-1625-2
Sami AS, Suat E, Alkis I, Karakus Y, Guler S (2021) The role of trace element, mineral, vitamin and total antioxidant status in women with habitual abortion. J Matern Fetal Neonatal Med 34(7):1055–1062.https://doi.org/10.1080/14767058.2019.1623872
Gomez-Lopez N, Guilbert LJ, Olson DM (2010) Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol 88(4):625–633.https://doi.org/10.1189/jlb.1209796
Pantos K, Grigoriadis S, Maziotis E, Pistola K, Xystra P, Pantou A, Kokkali G, Pappas A, Lambropoulou M, Sfakianoudis K, Simopoulou M (2022) The role of interleukins in recurrent implantation failure: a comprehensive review of the literature. Int J Mol Sci 23(4):2198. https://doi.org/10.3390/ijms23042198
Prabhudas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, Mccune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K (2015) Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16(4):328–334.https://doi.org/10.1038/ni.3131
von Rango U (2008) Fetal tolerance in human pregnancy–a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett 115(1):21–32.https://doi.org/10.1016/j.imlet.2007.09.014
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E (2009) van der Poel S (2009) The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology. Hum Reprod 24(11):2683–2687.https://doi.org/10.1093/humrep/dep343
Gu P, Yang X, Zhao X, Xu D (2021) The value of transvaginal 4-dimensional hysterosalpingo-contrast sonography in predicting the necessity of assisted reproductive technology for women with tubal factor infertility. Quant Imaging Med Surg 11(8):3698–3714.https://doi.org/10.21037/qims-20-1193
Luke B (2017) Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol 217(3):270–281.https://doi.org/10.1016/j.ajog.2017.03.012
Bitler MP, Schmidt L (2012) Utilization of infertility treatments: the effects of insurance mandates. Demography 49(1):125–149.https://doi.org/10.1007/s13524-011-0078-4
Okun N, Sierra S (2014) Pregnancy outcomes after assisted human reproduction. J Obstet Gynaecol Can 36(1):64–83.https://doi.org/10.1016/S1701-2163(15)30685-X
Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L (2015) Barfield WD (2018) Assisted reproductive technology surveillance - United States. MMWR Surveill Summ 67(3):1–28.https://doi.org/10.15585/mmwr.ss6703a1
Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L (2014) Barfield WD (2017) Assisted reproductive technology surveillance - United States. MMWR Surveill Summ 66(6):1–24.https://doi.org/10.15585/mmwr.ss6606a1
Sunderam S, Kissin DM, Zhang Y, Folger SG, Boulet SL, Warner L, Callaghan WM (2016) Barfield WD (2019) Assisted reproductive technology surveillance - United States. MMWR Surveill Summ 68(4):1–23.https://doi.org/10.15585/mmwr.ss6804a1
Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, Kroelinger CD (2017) Barfield WD (2020) Assisted reproductive technology surveillance - United States. MMWR Surveill Summ 69(9):1–20.https://doi.org/10.15585/mmwr.ss6909a1
Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, Kroelinger CD (2018) Barfield WD (2022) Assisted reproductive technology surveillance - United States. MMWR Surveill Summ 71(4):1–19.https://doi.org/10.15585/mmwr.ss7104a1
Li D, Liang C, Cao Y, Zhu D, Shen L, Zhang Z, Jiang T, Zhang Z, Zong K, Liu Y, Liang D, Cao Y, Ji D, Xu X (2022) The associations of serum metals concentrations with the intermediate and pregnancy outcomes in women undergoing in vitro fertilization (IVF). Ecotoxicol Environ Saf 233:113309.https://doi.org/10.1016/j.ecoenv.2022.113309
Jiang T, Hu Y, He S, Jiang R, Yao Y, Jin Z, Shen J, Tao F, Ji Y, Liang C (2022) Exposure to multiple toxic metals and the risk of early embryonic arrest among women undergoing assisted reproductive techniques. Environ Res 211:113072. https://doi.org/10.1016/j.envres.2022.113072
Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM (2017) Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin Rev Allergy Immunol 53(1):40–53.https://doi.org/10.1007/s12016-016-8596-x
Robertson SA, Care AS, Moldenhauer LM (2018) Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest 128(10):4224–4235.https://doi.org/10.1172/JCI122182
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13(6):397–411.https://doi.org/10.1038/nri3452
Palomo J, Dietrich D, Martin P, Palmer G, Gabay C (2015) The interleukin (IL)-1 cytokine family–balance between agonists and antagonists in inflammatory diseases. Cytokine 76(1):25–37.https://doi.org/10.1016/j.cyto.2015.06.017
van de Veerdonk FL, Netea MG (2013) New insights in the immunobiology of IL-1 family members. Front Immunol 4:167.https://doi.org/10.3389/fimmu.2013.00167
Kruessel JS, Huang HY, Wen Y, Kloodt AR, Bielfeld P, Polan ML (1997) Different pattern of interleukin-1 beta-(IL-1 beta), interleukin-1 receptor antagonist- (IL-1ra) and interleukin-1 receptor type I- (IL-1R tI) mRNA-expression in single preimplantation mouse embryos at various developmental stages. J Reprod Immunol 34(2):103–120.https://doi.org/10.1016/s0165-0378(97)00030-2
Simón C, Frances A, Piquette GN, El DI, Zurawski G, Dang W, Polan ML (1994) Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 134(2):521–528.https://doi.org/10.1210/endo.134.2.8299552
Spandorfer SD, Neuer A, Liu HC, Rosenwaks Z, Witkin SS (2003) Involvement of interleukin-1 and the interleukin-1 receptor antagonist in in vitro embryo development among women undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet 20(12):502–505.https://doi.org/10.1023/b:jarg.0000013650.76052.ae
Bonetti TC, Salomao R, Brunialti M, Braga DP, Borges EJ, Silva ID (2010) Cytokine and hormonal profile in serum samples of patients undergoing controlled ovarian stimulation: interleukin-1beta predicts ongoing pregnancy. Hum Reprod 25(8):2101–2106.https://doi.org/10.1093/humrep/deq171
Kreines FM, Nasioudis D, Minis E, Irani M, Witkin SS, Spandorfer S (2018) IL-1beta predicts IVF outcome: a prospective study. J Assist Reprod Genet 35(11):2031–2035.https://doi.org/10.1007/s10815-018-1296-0
Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM (2014) Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 5:253.https://doi.org/10.3389/fimmu.2014.00253
Revelli A, Delle PL, Casano S, Molinari E, Massobrio M, Rinaudo P (2009) Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 7:40.https://doi.org/10.1186/1477-7827-7-40
Cerkiene Z, Eidukaite A, Usoniene A (2008) Follicular fluid levels of interleukin-10 and interferon-gamma do not predict outcome of assisted reproductive technologies. Am J Reprod Immunol 59(2):118–126.https://doi.org/10.1111/j.1600-0897.2007.00552.x
Chaouat G, Dubanchet S, Ledee N (2007) Cytokines: important for implantation? J Assist Reprod Genet 24(11):491–505.https://doi.org/10.1007/s10815-007-9142-9
Alhilali M, Parham A, Attaranzadeh A, Amirian M, Azizzadeh M (2019) IL-5 in follicular fluid as a negative predictor of the intracytoplasmic sperm injection outcome. Cytokine 113:265–271.https://doi.org/10.1016/j.cyto.2018.07.016
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118.https://doi.org/10.1152/physrev.1993.73.1.79
Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, Woodruff TK (2012) A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 86(4):114.https://doi.org/10.1095/biolreprod.111.097253
Jeon Y, Yoon JD, Cai L, Hwang SU, Kim E, Zheng Z, Lee E, Kim DY, Hyun SH (2014) Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs. Theriogenology 82(6):866–874.https://doi.org/10.1016/j.theriogenology.2014.06.021
Kumar S, Mishra V, Thaker R, Gor M, Perumal S, Joshi P, Sheth H, Shaikh I, Gautam AK, Verma Y (2018) Role of environmental factors & oxidative stress with respect to in vitro fertilization outcome. Indian J Med Res 148(Suppl):S125–S133.https://doi.org/10.4103/ijmr.IJMR_1864_17
Hawk SN, Uriu-Hare JY, Daston GP, Jankowski MA, Kwik-Uribe C, Rucker RB, Keen CL (1998) Rat embryos cultured under copper-deficient conditions develop abnormally and are characterized by an impaired oxidant defense system. Teratology 57(6):310–320.https://doi.org/10.1002/(SICI)1096-9926(199806)57:6%3c310::AID-TERA4%3e3.0.CO;2-#
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10:49.https://doi.org/10.1186/1477-7827-10-49
Skalnaya MG, Tinkov AA, Lobanova YN, Chang JS, Skalny AV (2019) Serum levels of copper, iron, and manganese in women with pregnancy, miscarriage, and primary infertility. J Trace Elem Med Biol 56:124–130.https://doi.org/10.1016/j.jtemb.2019.08.009
Tulic L, Vidakovic S, Tulic I, Curcic M, Bulat Z (2019) Toxic metal and trace element concentrations in blood and outcome of in vitro fertilization in women. Biol Trace Elem Res 188(2):284–294.https://doi.org/10.1007/s12011-018-1421-z
Bernhardt ML, Kim AM, O’Halloran TV, Woodruff TK (2011) Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod 84(3):526–536.https://doi.org/10.1095/biolreprod.110.086488
Kim AM, Vogt S, O’Halloran TV, Woodruff TK (2010) Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 6(9):674–681.https://doi.org/10.1038/nchembio.419
Tian X, Diaz FJ (2012) Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 153(2):873–886.https://doi.org/10.1210/en.2011-1599
Feijo G, Jantsch J, Correia LL, Eller S, Furtado-Filho OV, Giovenardi M, Porawski M, Braganhol E, Guedes RP (2022) Neuroinflammatory responses following zinc or branched-chain amino acids supplementation in obese rats. Metab Brain Dis 37(6):1875–1886.https://doi.org/10.1007/s11011-022-00996-5
Ju C, Hwang S, Cho GS, Kondaji G, Song S, Prather PL, Choi Y, Kim WK (2013) Differential anti-ischemic efficacy and therapeutic time window of trans- and cis-hinokiresinols: stereo-specific antioxidant and anti-inflammatory activities. Neuropharmacology 67:465–475.https://doi.org/10.1016/j.neuropharm.2012.12.006
Alrashed M, Tabassum H, Almuhareb N, Almutlaq N, Alamro W, Alanazi ST, Alenazi FK, Alahmed LB, Al AM, Alenzi ND (2021) Assessment of DNA damage in relation to heavy metal induced oxidative stress in females with recurrent pregnancy loss (RPL). Saudi J Biol Sci 28(9):5403–5407.https://doi.org/10.1016/j.sjbs.2021.05.068
Tabassum H, Alrashed M, Malik A, Alanazi ST, Alenzi ND, Ali MN, Aljaser FS, Altoum GH, Hijazy SM, Alfadhli RA, Alrashoudi R, Akhtar S (2022) A unique investigation of thallium, tellurium, osmium, and other heavy metals in recurrent pregnancy loss: a novel approach. Int J Gynaecol Obstet.https://doi.org/10.1002/ijgo.14390
Yildirim E, Derici MK, Demir E, Apaydin H, Kocak O, Kan O, Gorkem U (2019) Is the concentration of cadmium, lead, mercury, and selenium related to preterm birth? Biol Trace Elem Res 191(2):306–312.https://doi.org/10.1007/s12011-018-1625-2
Zachara BA (2018) Selenium in complicated pregnancy. A review Adv Clin Chem 86:157–178.https://doi.org/10.1016/bs.acc.2018.05.004
Acknowledgements
The authors thank all the medical staff in the First Afflicted Hospital of Anhui Medical University in Anhui, China for their work in recruiting subjects and collecting specimens. The authors are grateful to the Scientific Research Center in Preventive Medicine, School of Public Health, Anhui Medical University for the technical support in our experiment.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC–82173532, NSFC–U20A20350, NSFC–81971455, NSFC–81871216 and NSFC–81601345), the Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (gxyq2021173), the Postdoctoral Research Foundation of China (2021M700181), the University Natural Science Research Project of Anhui Province (KJ2020A0203), the National Key Research and Development Program (2018YFC1004201, 2016YFC1000204).
Author information
Authors and Affiliations
Contributions
Yunxia Cao, Dongmei Ji, Yanli Ji, and Chunmei Liang figured out the conception of this study; Yuan Hu, Dongyang Zhang, Shitao He, and Tingting Jiang tested the blood samples; Mengzhu Li, Xinyu Yue, and Guiying Luo collected the blood samples; Yuan Hu, Dongyang Zhang, Qing Zhang, and Tao Yin analyzed the data and wrote the manuscript; Fangbiao Tao, Chunmei Liang, Yanli Ji, Yunxia Cao, Guiying Luo, and Dongmei Ji offered proposals and provided financial support. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Zhang, D., Zhang, Q. et al. Serum Cu, Zn and IL–1β Levels May Predict Fetal Miscarriage Risk After IVF Cycles: A Nested Case–Control Study. Biol Trace Elem Res 201, 5561–5574 (2023). https://doi.org/10.1007/s12011-023-03621-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03621-3