Abstract
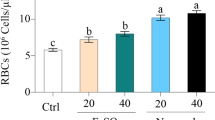
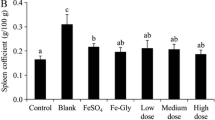
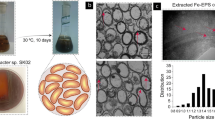
The objective of this study was to investigate the effects of iron nanoliposomes on iron supplementation and toxicity in SD rats induced by a low-iron diet. The size and infrared spectroscopy of a liposomal oral delivery system were investigated. The particle size of nanoliposomes embedded with chelates was increased. Infrared spectra proved that peptides-iron and blank nanoliposomes were bonded by interaction forces, including the fracture of hydrogen bonds, C = C bonds, hydrophobic interaction, and C-N bonds. We found that iron supplementation chelates had a certain protective effect on viscera after being embedded by nanoliposomes. After 10 days of treatment, the concentration of hemoglobin could be gradually increased. Nanoliposome encapsulated peptides-iron has a better effect than other groups. At the same time, SOD, MDA, and CAT reached normal levels after 20 days. Histological results showed that the sections of the nanoliposomes groups were clearer than those of the other groups. There was a little inflammation in the liver without obvious pathological changes, which also proved that the iron chelates embedded by nanoliposomes had no obvious side effects on iron supplementation in rats. Nanoliposome encapsulated peptides-iron has a small side effect and a significant curative effect of iron supplementation. It maybe has a good application prospect in the clinical medical field.








Similar content being viewed by others
Data Availability
All data reported in this study are available upon request by contact with the corresponding author.
References
Girelli D, Marchi G, Busti F, Vianello A (2021) Iron metabolism in infections: focus on COVID-19. Semin Hematol 58(3):182–187. https://doi.org/10.1053/j.seminhematol.2021.07.001
Sheftel AD, Mason AB (1820) Ponka P (2012) The long history of iron in the Universe and in health and disease. Biochim Biophys Acta 3:161–187. https://doi.org/10.1016/j.bbagen.2011.08.002
Elshemy M (2018) Iron oxide nanoparticles versus ferrous sulfate in treatment of iron deficiency anemia in rats. Egyptian J Vet Sci 49(2):103–109. https://doi.org/10.21608/ejvs.2018.3855.1039
Percy L, Mansour D, Fraser I (2017) Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol 40:55–67. https://doi.org/10.1016/j.bpobgyn.2016.09.007
Maria H (2017) Thalassemia: yesterday, today, tomorrow. Am J Hematol 92(6):490–492. https://doi.org/10.1002/ajh.24744
Wood JC, Cohen AR, Pressel SL, Aygun B, Imran H, Luchtman-Jones L, Thompson AA, Fuh B, Schultz WH, Davis BR (2016) Organ iron accumulation in chronically transfused children with sickle cell anaemia: baseline results from the TWiTCH trial. Br J Haematol 172(1):122–130. https://doi.org/10.1111/bjh.13791
Helal MG, El-Kashef DH (2020) Krill oil alleviates oxidative stress, iron accumulation and fibrosis in the liver and spleen of iron-overload rats. Environ Sci Pollut Res 27(4):3950–3961. https://doi.org/10.1007/s11356-019-06983-1
Salama SA, Kabel AM (2020) Taxifolin ameliorates iron overload-induced hepatocellular injury: modulating PI3K/AKT and p38 MAPK signaling inflammatory response and hepatocellular regeneration. Chem-Biol Interact 330:10923010. https://doi.org/10.1016/j.cbi.2020.109230
Kim EY, Ham SK, Bradke D, Ma Q, Han O (2011) Ascorbic acid offsets the inhibitory effect of bioactive dietary polyphenolic compounds on transepithelial iron transport in Caco-2 intestinal cells. J Nutr 141(5):828–834. https://doi.org/10.3945/jn.110.134031
Salama SA, Elshafey MM (2022) Cross-talk between PPARγ, NF-κB, and p38 MAPK signaling mediates the ameliorating effects of bergenin against the iron overload-induced hepatotoxicity. Chem Biol Interact 368:110207. https://doi.org/10.1016/j.cbi.2022.110207
Guo L, Harnedy PA, Li B, Hou H, Zhang Z, Zhao X, FitzGerald RJ (2014) Food protein-derived chelating peptides: biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci Technol 37(2):92–105. https://doi.org/10.1016/j.tifs.2014.02.007
Caetano-Silva ME, Cilla A, Bertoldo-Pacheco MT, Netto FM, Alegría A (2018) Evaluation of in vitro iron bioavailability in free form and as whey peptide-iron complexes. J Food Compos Anal 68:95–100. https://doi.org/10.1016/j.jfca.2017.03.010
Argyri K, Miller DD, Glahn RP, Zhu L, Kapsokefalou M (2007) Peptides isolated from in vitro digests of milk enhance iron uptake by Caco-2 cells. J Agric Food Chem 55(25):10221–10225. https://doi.org/10.1021/jf0727387
Ou K, Liu Y, Zhang L, Yang X, Huang Z, Nout M, Liang J (2010) Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 cells. J Agric Food Chem 58(8):4894. https://doi.org/10.1021/jf100055y
Li Y, Jiang H, Huang G (2017) Protein hydrolysates as promoters of non-haem iron absorption. Nutrients 9(6):609. https://doi.org/10.3390/nu9060609
Chen C, Sun-Waterhouse D, Zhao J, Zhao M, Waterhouse GIN, Sun W (2021) Soybean protein isolate hydrolysates-liposomes interactions under oxidation: mechanistic insights into system stability. Food Hydrocolloid 112:106336. https://doi.org/10.1016/j.foodhyd.2020.106336
Chotphruethipong L, Battino M, Benjakul S (2020) Effect of stabilizing agents on characteristics, antioxidant activities and stability of liposome loaded with hydrolyzed collagen from defatted Asian sea bass skin. Food Chem 328:127127. https://doi.org/10.1016/j.foodchem.2020.127127
Ge J, Han B, Hu H, Liu J, Liu Y (2015) Epigallocatechin-3-O-gallate protects against hepatic damage and testicular toxicity in male mice exposed to di-(2-ethylhexyl) phthalate. J Med Food 18(7):753–761. https://doi.org/10.1089/jmf.2014.3247
Chen M, Chen C, Wu J, Bi J, Jiang H, Huang G (2021) Comparison of ferrous ion chelating properties of collagen peptides from dried and fresh cod skin. Am J Biochem Biotechnol 17(3):290–301. https://doi.org/10.3844/ajbbsp.2021.290.301
Liang J, Tian Y-X, Fu L-M, Wang T-H, Li H-J, Wang P, Han R-M, Zhang J-P, Skibsted LH (2008) Daidzein as an antioxidant of lipid: effects of the microenvironment in relation to chemical structure. J Agric Food Chem 56(21):10376–10383. https://doi.org/10.1021/jf801907m
Zhang M, Liu W, Li G (2009) Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem 115(3):826–831. https://doi.org/10.1016/j.foodchem.2009.01.006
Urso K, Leal Martínez-Bujanda J, del Prado JM (2021) Iron protein succinylate in the management of iron deficiency anemia: a comparative study with ferrous sulphate at low and high therapeutic doses. Nutrients 13(3):968. https://doi.org/10.3390/nu13030968
Hashemi B, Madadlou A, Salami M (2017) Functional and in vitro gastric digestibility of the whey protein hydrogel loaded with nanostructured lipid carriers and gelled via citric acid-mediated crosslinking. Food Chem 237:23–29. https://doi.org/10.1016/j.foodchem.2017.05.077
Bai C, Peng H, Xiong H, Liu Y, Zhao L, Xiao X (2011) Carboxymethylchitosan-coated proliposomes containing coix seed oil: characterisation, stability and in vitro release evaluation. Food Chem 129(4):1695–1702. https://doi.org/10.1016/j.jff.2013.11.012
Abaee A, Madadlou A (2016) Niosome-loaded cold-set whey protein hydrogels. Food Chem 196:106–113. https://doi.org/10.1016/j.foodchem.2015.09.037
Bagheri L, Mada D, Lou A, Yarmand M, Mousavi ME (2014) Potentially bioactive and caffeine-loaded peptidic sub-micron and nanoscalar particles. J Funct Foods 6:462–469. https://doi.org/10.1016/j.jff.2013.11.012
Wu L, Zou Y, Miao Y, Zhang J, Zhu S, Zeng M, Wu H (2019) Dietary gelatin enhances non-heme iron absorption possibly via regulation of systemic iron homeostasis in rats. J Funct Foods 59:272–280. https://doi.org/10.1016/j.jff.2019.06.005
Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematol 105(2):260. https://doi.org/10.3324/haematol.2019.232124
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta 1767(9):1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Viana-Baracioli L, Junior N, Junior OR, Mattos LC, Bonini-Domingos CR (2011) Comparison of oxidative stress and the frequency of polymorphisms in the HFE gene between hemoglobin S trait blood donors and sickle cell disease patients. Genet Mol Res 10(4):3446. https://doi.org/10.4238/2011.December.8.4
Yuan L, Geng L, Ge L, Yu P, Duan X, Chen J, Chang Y (2013) Effect of iron liposomes on anemia of inflammation. Int J Pharm 454(1):82–89. https://doi.org/10.1016/j.ijpharm.2013.06.078
Jia N, Qiao H, Zhu W, Zhu M, Meng Q, Lu Q, Zu Y (2019) Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int J Biol Macromol 132:213–221. https://doi.org/10.1016/j.ijbiomac.2019.03.196
Eloy JO, Claro de Souza M, Petrilli R, Barcellos JP, Lee RJ, Marchetti JM (2014) Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B Biointerfaces 123:345–363. https://doi.org/10.1016/j.colsurfb.2014.09.029
Shilova E, Koltakov I, Kannykin S, Artyukhov V (2022) Inclusion of magnetite nanoparticles stabilized with cetyltrimethylammonium bromide in soy lecithin-based liposomes. Biophysics 67(3):435–439. https://doi.org/10.1134/S0006350922030216
Vrignaud S, Benoit J-P, Saulnier P (2011) Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 32(33):8593–8604. https://doi.org/10.1016/j.biomaterials.2011.07.057
Funding
This research is supported by the Science and Technology Project of Zhejiang, Province, China [grant number LGN19C200018] and the Fundamental Research Funds for the Provincial Universities of Zhejiang [No 2020YW34].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Methodology and validation were performed by Mengqian Chen and Guangrong Huang. The first draft of the manuscript was written by Mengqian Chen and Cen Chen, visualization and chart making was performed by Yuhang Zhang, Han Jiang, YiZhou Fang, writing-reviewing and editing were performed by Guangrong Huang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animals were kept in a pathogen-free environment and fed ad lib. The procedures for care and use of animals were approved by the Ethics Committee of the China Jiliang University and all applicable institutional and governmental regulations concerning the ethical use of animals were followed.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Chen, C., Zhang, Y. et al. Effects of Iron-Peptides Chelate Nanoliposomes on Iron Supplementation in Rats. Biol Trace Elem Res 201, 4508–4517 (2023). https://doi.org/10.1007/s12011-022-03539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03539-2