Abstract
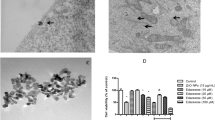
Zinc is an essential trace element, which plays an important role in multiple biological activities. However, excessive exposure to zinc can cause toxic damage to living organism. Here, we investigated the relationship between oxidative stress and mitochondrial dynamics in the zinc-induced cytotoxicity. Results showed that excess exposure to zinc could significantly reduce cell viability and induce cell vacuolation in PK-15 cells. Additionally, zinc exposure caused mitochondrial dynamics disorder, manifested as mitochondrial fission, and the elevated mRNA level of Drp1 and downregulated mRNA levels of OPA1, Mfn1, and Mfn2. Meanwhile, zinc could induce oxidative damage, evidenced by the increasing levels of hydrogen peroxide, malondialdehyde, lipid peroxidation, oxidized form of nicotinamide adenine dinucleotide phosphate/nicotinamide adenine dinucleotide phosphate, oxidized glutathione/glutathione, superoxide dismutase activity, and the mRNA expression of SOD-1 and NOQ1, and decreasing levels of catalase activity, glutathione peroxidase activity, glutathione reductase activity, and the mRNA expression of CAT, and GPX1. Interestingly, N-acetyl-L-cysteine, an inhibitor for oxidative stress, could reduce the mitochondrial fission under zinc treatment. Besides, Mdivi-1, a mitochondrial fission inhibitor, could relieve oxidative stress caused by excess zinc. In general, these results suggested that mitochondrial fission and oxidative stress induced by zinc were interrelated in PK-15 cells, which is conducive to explore the new mechanism of zinc toxicity and proposes a theoretical foundation for selecting effective drugs to alleviate the toxic effects caused by zinc.






Similar content being viewed by others
References
Baarz BR, Laurentius T, Wolf J, Wessels I, Bollheimer LC, Rink L (2022) Short-term zinc supplementation of zinc-deficient seniors counteracts CREMalpha - mediated IL-2 suppression. Immun Ageing 19(1):40. https://doi.org/10.1186/s12979-022-00295-8
Kataba A, Nakayama S, Yohannes YB, Toyomaki H, Nakata H, Ikenaka Y, Ishizuka M (2021) Effects of zinc on tissue uptake and toxicity of lead in Sprague Dawley rat. J Vet Med Sci 83(11):1674–1685. https://doi.org/10.1292/jvms.20-0684
Hill GM, Shannon MC (2019) Copper and zinc nutritional issues for agricultural animal production. Biol Trace Elem Res 188(1):148–159. https://doi.org/10.1007/s12011-018-1578-5
Abdel-Azeem HH, Osman GY (2021) Oxidative stress and histopathological effect of zinc oxide nanoparticles on the garden snail Helix aspersa. Environ Sci Pollut Res Int 28(8):9913–9920. https://doi.org/10.1007/s11356-020-11438-z
Yang Q, Fang Y, Zhang C, Liu X, Wu Y, Zhang Y, Yang J, Yong K (2022) Exposure to zinc induces lysosomal-mitochondrial axis-mediated apoptosis in PK-15 cells. Ecotoxicol Environ Saf 241:113716. https://doi.org/10.1016/j.ecoenv.2022.113716
Liang J, Cao R, Wang X, Zhang Y, Wang P, Gao H, Li C, Yang F, Zeng R, Wei P, Li D, Li W, Yang W (2017) Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res 27(3):329–351. https://doi.org/10.1038/cr.2016.159
Li Q, Liao J, Chen W, Zhang K, Li H, Ma F, Zhang H, Han Q, Guo J, Li Y, Hu L, Pan J, Tang Z (2022) NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic Biol Med 187:158–170. https://doi.org/10.1016/j.freeradbiomed.2022.05.024
Wu Z, Wang H, Fang S, Xu C (2018) Roles of endoplasmic reticulum stress and autophagy on H2O2induced oxidative stress injury in HepG2 cells. Mol Med Rep 18(5):4163–4174. https://doi.org/10.3892/mmr.2018.9443
Wu S, Pan L, Liao H, Yao W, Shen N, Chen C, Liu D, Ge M (2020) High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci 253:117539. https://doi.org/10.1016/j.lfs.2020.117539
Yi X, Guo W, Shi Q, Yang Y, Zhang W, Chen X, Kang P, Chen J, Cui T, Ma J, Wang H, Guo S, Chang Y, Liu L, Jian Z, Wang L, Xiao Q, Li S, Gao T, Li C (2019) SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics 9(6):1614–1633. https://doi.org/10.7150/thno.30398
Giacomello M, Pyakurel A, Glytsou C, Scorrano L (2020) The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol 21(4):204–224. https://doi.org/10.1038/s41580-020-0210-7
Mishra P, Chan DC (2016) Metabolic regulation of mitochondrial dynamics. J Cell Biol 212(4):379–387. https://doi.org/10.1083/jcb.201511036
Wai T, Langer T (2016) Mitochondrial dynamics and metabolic regulation. Trends Endocrinol Metab 27(2):105–117. https://doi.org/10.1016/j.tem.2015.12.001
Cao YP, Zheng M (2019) Mitochondrial dynamics and inter-mitochondrial communication in the heart. Arch Biochem Biophys 663:214–219. https://doi.org/10.1016/j.abb.2019.01.017
Xie JH, Li YY, Jin J (2020) The essential functions of mitochondrial dynamics in immune cells. Cell Mol Immunol 17(7):712–721. https://doi.org/10.1038/s41423-020-0480-1
Gilbert R, Peto T, Lengyel I, Emri E (2019) Zinc nutrition and inflammation in the aging retina. Mol Nutr Food Res 63(15):e1801049. https://doi.org/10.1002/mnfr.201801049
Zackular JP, Skaar EP (2018) The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microbes 9(5):469–476. https://doi.org/10.1080/19490976.2018.1448354
Jin Z, Ding S, Sun Q, Gao S, Fu Z, Gong M, Lin J, Wang D, Wang Y (2019) High resolution spatiotemporal sampling as a tool for comprehensive assessment of zinc mobility and pollution in sediments of a eutrophic lake. J Hazard Mater 364:182–191. https://doi.org/10.1016/j.jhazmat.2018.09.067
Yang Y, Yu Y, Zhou R, Yang Y, Bu Y (2021) The effect of combined exposure of zinc and nickel on the development of zebrafish. J Appl Toxicol 41(11):1765–1778. https://doi.org/10.1002/jat.4159
Adedara IA, Adegbosin AN, Abiola MA, Odunewu AA, Owoeye O, Owumi SE, Farombi EO (2020) Neurobehavioural and biochemical responses associated with exposure to binary waterborne mixtures of zinc and nickel in rats. Environ Toxicol Pharmacol 73:103294. https://doi.org/10.1016/j.etap.2019.103294
Liu Z, Chen B, Li X, Wang LA, Xiao H, Liu D (2019) Toxicity assessment of artificially added zinc, selenium, and strontium in water. Sci Total Environ 670:433–438. https://doi.org/10.1016/j.scitotenv.2019.03.259
Kleele T, Rey T, Winter J, Zaganelli S, Mahecic D, Perreten LH, Ruberto FP, Nemir M, Wai T, Pedrazzini T, Manley S (2021) Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 593(7859):435–439. https://doi.org/10.1038/s41586-021-03510-6
Yapa N, Lisnyak V, Reljic B, Ryan MT (2021) Mitochondrial dynamics in health and disease. Febs Lett 595(8):1184–1204. https://doi.org/10.1002/1873-3468.14077
Li X, Bai Y, Huo H, Wu H, Liao J, Han Q, Zhang H, Hu L, Li Y, Pan J, Tang Z, Guo J (2022) Long-term copper exposure induces mitochondrial dynamics disorder and mitophagy in the cerebrum of pigs. Biol Trace Elem Res. https://doi.org/10.1007/s12011-022-03224-4
Jin JY, Wei XX, Zhi XL, Wang XH, Meng D (2021) Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin 42(5):655–664. https://doi.org/10.1038/s41401-020-00518-y
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C, Lv Y, Wu R (2019) Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol 20:296–306. https://doi.org/10.1016/j.redox.2018.10.019
Liao J, Yang F, Bai Y, Yu W, Qiao N, Han Q, Zhang H, Guo J, Hu L, Li Y, Pan J, Tang Z (2021) Metabolomics analysis reveals the effects of copper on mitochondria-mediated apoptosis in kidney of broiler chicken (Gallus gallus). J Inorg Biochem 224:111581. https://doi.org/10.1016/j.jinorgbio.2021.111581
Wu Y, Yang F, Zhou G, Wang Q, Xing C, Bai H, Yi X, Xiong Z, Yang S, Cao H (2022) Molybdenum and cadmium co-induce mitochondrial quality control disorder via FUNDC1-mediated mitophagy in sheep kidney. Front Vet Sci 9:842259. https://doi.org/10.3389/fvets.2022.842259
Xiong Z, Xing C, Xu T, Yang Y, Liu G, Hu G, Cao H, Zhang C, Guo X, Yang F (2021) Vanadium induces oxidative stress and mitochondrial quality control disorder in the heart of ducks. Front Vet Sci 8:756534. https://doi.org/10.3389/fvets.2021.756534
Kaarniranta K, Kajdanek J, Morawiec J, Pawlowska E, Blasiak J (2018) PGC-1alpha protects RPE cells of the aging retina against oxidative stress-induced degeneration through the regulation of senescence and mitochondrial quality control. The significance for AMD pathogenesis. Int J Mol Sci 19(8). https://doi.org/10.3390/ijms19082317
Ayna A (2021) Caffeic acid prevents hydrogen peroxide-induced oxidative damage in SH-SY5Y cell line through mitigation of oxidative stress and apoptosis. Bratisl Lek Listy 122(2):120–124. https://doi.org/10.4149/BLL_2021_018
Yang F, Pei R, Zhang Z, Liao J, Yu W, Qiao N, Han Q, Li Y, Hu L, Guo J, Pan J, Tang Z (2019) Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol In Vitro 54:310–316. https://doi.org/10.1016/j.tiv.2018.10.017
Huo H, Wang S, Bai Y, Liao J, Li X, Zhang H, Han Q, Hu L, Pan J, Li Y, Tang Z, Guo J (2021) Copper exposure induces mitochondrial dynamic disorder and oxidative stress via mitochondrial unfolded protein response in pig fundic gland. Ecotoxicol Environ Saf 223:112587. https://doi.org/10.1016/j.ecoenv.2021.112587
Pan YX, Luo Z, Zhuo MQ, Wei CC, Chen GH, Song YF (2018) Oxidative stress and mitochondrial dysfunction mediated Cd-induced hepatic lipid accumulation in zebrafish Danio rerio. Aquat Toxicol 199:12–20. https://doi.org/10.1016/j.aquatox.2018.03.017
Liao J, Yang F, Chen H, Yu W, Han Q, Li Y, Hu L, Guo J, Pan J, Liang Z, Tang Z (2019) Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotoxicol Environ Saf 185:109710. https://doi.org/10.1016/j.ecoenv.2019.109710
Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y, Hu Y, Huang Y (2021) SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev 2021:9265016. https://doi.org/10.1155/2021/9265016
Duan C, Wang L, Zhang J, Xiang X, Wu Y, Zhang Z, Li Q, Tian K, Xue M, Liu L, Li T (2020) Mdivi-1 attenuates oxidative stress and exerts vascular protection in ischemic/hypoxic injury by a mechanism independent of Drp1 GTPase activity. Redox Biol 37:101706. https://doi.org/10.1016/j.redox.2020.101706
Rexius-Hall ML, Khalil NN, Andres AM, Mccain ML (2020) Mitochondrial division inhibitor 1 (mdivi-1) increases oxidative capacity and contractile stress generated by engineered skeletal muscle. Faseb J 34(9):11562–11576. https://doi.org/10.1096/fj.201901039RR
Aparicio-Trejo OE, Reyes-Fermin LM, Briones-Herrera A, Tapia E, Leon-Contreras JC, Hernandez-Pando R, Sanchez-Lozada LG, Pedraza-Chaverri J (2019) Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med 130:379–396. https://doi.org/10.1016/j.freeradbiomed.2018.11.005
Ali M, Tabassum H, Alam MM, Parvez S (2022) N-acetyl-L-cysteine ameliorates mitochondrial dysfunction in ischemia/reperfusion injury via attenuating Drp-1 mediated mitochondrial autophagy. Life Sci 293:120338. https://doi.org/10.1016/j.lfs.2022.120338
Funding
This study was sponsored by Natural Science Foundation of Chongqing (grant number: cstc2021jcyj-msxmX1210), China, the Educational Reform Project of Chongqing Municipal Education Commission (grant number: Z213122), and the Science and Technology Research Program of Chongqing Municipal Education Commission (grant number: KJQN202003504; KJQN 202003509; KJQN202203511).
Author information
Authors and Affiliations
Contributions
Qingwen Yang, Junjie Yang, and Kang Yong wrote the main manuscript text, Yi Zhang, Yue Li, Da Ao, Peng Zhong and Xuesong Liu prepared figures. Qingwen Yang and Kang Yong provide the project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Qingwen Yang and Junjie Yang share the first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Q., Yang, J., Liu, X. et al. Crosstalk Between the Mitochondrial Dynamics and Oxidative Stress in Zinc-induced Cytotoxicity. Biol Trace Elem Res 201, 4419–4428 (2023). https://doi.org/10.1007/s12011-022-03504-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03504-z