Abstract
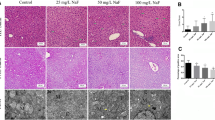
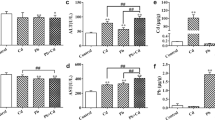
Aluminum (Al) exposure can lead to different degrees of damage to various organ systems of the body. It has been previously revealed that Al exposure can damage the liver, causing liver dysfunction. However, the specific mechanism remains unclear. This research aims to uncover the damaging effect of Al exposure on rat liver and to demonstrate the role of autophagy and apoptosis in this effect. Thirty-two Wistar rats were randomly divided into the control group (C group), low-dose Al exposure group (L group), middle-dose Al exposure group (M group), and high-dose Al exposure group (H group) (n = 8). The rats, respectively, received intraperitoneal injections of 0, 5, 10, and 20 mg/kg·day AlCl3 solution for 4 weeks (5 times/week). After the experiment, changes in the ultrastructure and autolysosome in rat liver were observed; the liver function, apoptosis rate, as well as levels of apoptosis-associated proteins and autophagy-associated proteins were detected. The results indicated that Al exposure damaged rat liver function and structure and resulted in an increase in autolysosomes. TUNEL staining revealed an elevated number of apoptotic hepatocytes after Al exposure. Moreover, we found from Western blotting that the levels of autophagy-associated proteins Beclin1 and LC3-II were increased; apoptotic protein Caspase-3 level was elevated and the Bcl-2/Bax ratio was reduced. Our research suggested that Al exposure can lead to high autophagy and apoptosis levels of rat hepatocytes, accompanied by hepatocyte injury and impaired liver function. This study shows that autophagy and apoptosis pathways participate in Al toxication-induced hepatocyte injury.






Similar content being viewed by others
Data Availability
The data presented in this study are available on request from the corresponding author and the first author.
References
Kim H, Lim KY, Kang J, Park JW, Park SH (2020) Macrophagic myofasciitis and subcutaneous pseudolymphoma caused by aluminium adjuvants. Sci Rep 10(1):11834. https://doi.org/10.1038/s41598-020-68849-8
Eraslan G, Sarıca ZS, Bayram L, Tekeli MY, Kanbur M, Karabacak M (2017) The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ Sci Pollut Res Int 24(36):27931–27941. https://doi.org/10.1007/s11356-017-0232-7
Martinez CS, Piagette JT, Escobar AG, Martin A, Palacios R, Pecanha FM, Vassallo DV, Exley C, Alonso MJ, Miguel M, Salaices M, Wiggers GA (2017) Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: a concerted action of NAD(P)H oxidase and COX-2. Toxicol 390:10–21. https://doi.org/10.1016/j.tox.2017.08.004
Xiao B, Cui Y, Li B, Zhang J, Zhang X, Song M, Li Y (2022) ROS antagonizes the protection of Parkin-mediated mitophagy against aluminum-induced liver inflammatory injury in mice. Food Chem Toxicol 165:113126. https://doi.org/10.1016/j.fct.2022.113126
Hassan SA, Kadry MO (2021) Neurodegenerative and hepatorenal disorders induced via aluminum chloride in murine system: impact of β-secretase, MAPK, and KIM. Biol Trace Elem Res 199(1):227–236. https://doi.org/10.1007/s12011-020-02132-9
Sajjad S, Malik H, Saeed L, Hashim I, Farooq U, Manzoor F (2019) Synergistic potential of propolis and vitamin e against sub-acute toxicity of AlCl(3) in albino mice: in vivo study. Physiol Res 68(1):67–74. https://doi.org/10.33549/physiolres.933863
Klein GL (2019) Aluminum toxicity to bone: a multisystem effect? Osteoporosis sarcopenia 5(1):2–5. https://doi.org/10.1016/j.afos.2019.01.001
Sieg H, Ellermann AL, Maria Kunz B, Jalili P, Burel A, Hogeveen K, Böhmert L, Chevance S, Braeuning A, Gauffre F, Fessard V, Lampen A (2019) Aluminum in liver cells—the element species matters. Nanotoxicol 13(7):909–922. https://doi.org/10.1080/17435390.2019.1593542
Yang X, Zhang J, Ji Q, Wang F, Song M, Li Y (2018) Autophagy protects MC3T3-E1 cells upon aluminum-induced apoptosis. Biol Trace Elem Res 185(2):433–439. https://doi.org/10.1007/s12011-018-1264-7
Mejías-Peña Y, Estébanez B, Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, de Paz JA, González-Gallego J, Cuevas MJ (2017) Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging 9(2):408–418. https://doi.org/10.18632/aging.101167
Frietze KK, Brown AM, Das D, Franks RG, Cunningham JL, Hayward M, Nickels JT Jr (2022) Lipotoxicity reduces DDX58/Rig-1 expression and activity leading to impaired autophagy and cell death. Autophagy 18(1):142–160. https://doi.org/10.1080/15548627.2021.1920818
Stewart T, Kallash M, Vehaskari VM, Hodgeson SM, Aviles DH (2019) Increased autophagy and apoptosis in the kidneys of intrauterine growth restricted rats. Fetal Pediatr Pathol 38(3):185–194. https://doi.org/10.1080/15513815.2018.1564160
Cheraghi E, Golkar A, Roshanaei K, Alani B (2017) Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in Wistar rats: ameliorative effects of curcumin. Int J Fertil Steril 11(3):166–175. https://doi.org/10.22074/ijfs.2017.4859
Li Z, Zhao G, Qian S, Yang Z, Chen X, Chen J, Cai C, Liang X, Guo J (2012) Cerebrovascular protection of β-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol 144(2):305–312. https://doi.org/10.1016/j.jep.2012.09.013
De A, Ghosh S, Chakrabarti M, Ghosh I, Banerjee R, Mukherjee A (2020) Effect of low-dose exposure of aluminium oxide nanoparticles in Swiss albino mice: Histopathological changes and oxidative damage. Toxicol Ind Health 36(8):567–579. https://doi.org/10.1177/0748233720936828
Salem AM, Mohammaden TF, Ali MAM, Mohamed EA, Hasan HF (2016) Ellagic and ferulic acids alleviate gamma radiation and aluminium chloride-induced oxidative damage. Life Sci 160:2–11. https://doi.org/10.1016/j.lfs.2016.07.006
Abu-Elfotuh K, Hussein FH, Abbas AN, Al-Rekabi MD, Barghash SS, Zaghlool SS, El-Emam SZ (2022) Melatonin and zinc supplements with physical and mental activities subside neurodegeneration and hepatorenal injury induced by aluminum chloride in rats: inclusion of GSK-3β-Wnt/β-catenin signaling pathway. Neurotoxicol 91:69–83. https://doi.org/10.1016/j.neuro.2022.05.002
Gao HT, Cheng WZ, Xu Q, Shao LX (2017) Dietary restriction reduces blood lipids and ameliorates liver function of mice with hyperlipidemia. J Huazhong Univ Sci Technolog Med Sci 37(1):79–86. https://doi.org/10.1007/s11596-017-1698-8
Al-Hazmi MA, Rawi SM, Hamza RZ (2021) Biochemical, histological, and neuro-physiological effects of long-term aluminum chloride exposure in rats. Metab Brain Dis 36(3):429–436. https://doi.org/10.1007/s11011-020-00664-6
Salimi A, Shabani M, Aylar EM (2022) Inhibition of mitochondrial permeability transition pore and antioxidant effect of Delta-9-tetrahydrocannabinol reduces aluminium phosphide-induced cytotoxicity and dysfunction of cardiac mitochondria. Pesticide Biochem Physiol 184:105117. https://doi.org/10.1016/j.pestbp.2022.105117
Al-Kahtani M, Abdel-Daim MM, Sayed AA, El-Kott A, Morsy K (2020) Curcumin phytosome modulates aluminum-induced hepatotoxicity via regulation of antioxidant, Bcl-2, and caspase-3 in rats. Environ Sci Pollut Res Int 27(17):21977–22185. https://doi.org/10.1007/s11356-020-08636-0
Weisser K, Göen T (2019) Aluminium in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Arch Toxicol 93(10):2787–2796. https://doi.org/10.1007/s00204-019-02561-z
El-Demerdash FM, Baghdadi HH, Ghanem NF, Mhanna ABA (2020) Nephroprotective role of bromelain against oxidative injury induced by aluminium in rats. Environ Toxicol Pharmacol 80:103509. https://doi.org/10.1016/j.etap.2020.103509
Rizvi SH, Parveen A, Ahmad I, Ahmad I, Verma AK, Arshad M, Mahdi AA (2016) Aluminum activates PERK-EIF2alpha signaling and inflammatory proteins in human neuroblastoma SH-SY5Y cells. Biol Trace Elem Res 172(1):108–119. https://doi.org/10.1007/s12011-015-0553-7
Bañuls C, de Marañón AM, Castro-Vega I, López-Doménech S, Escribano-López I, Salom C, Veses S (2019) Hernández-Mijares A (2019) Role of endoplasmic reticulum and oxidative stress parameters in the pathophysiology of disease-related malnutrition in leukocytes of an outpatient population. Nutrients 11(8):1838. https://doi.org/10.3390/nu11081838
Karna KK, Choi BR, Kim MJ, Kim HK, Park JK (2019) The effect of Schisandra chinensis Baillon on cross-talk between oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in testes of varicocele-induced SD rat. Int J Mol Sci 20(22):5785. https://doi.org/10.3390/ijms20225785
Zhang Y, Li S, Li J, Han L, He Q, Wang R, Wang X, Liu K (2018) Developmental toxicity induced by PM2.5 through endoplasmic reticulum stress and autophagy pathway in zebrafish embryos. Chemosphere 197:611–621. https://doi.org/10.1016/j.chemosphere.2018.01.092
Zou J, Fei Q, Xiao H, Wang H, Liu K, Liu M, Zhang H, Xiao X, Wang K (2019) VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol 234(10):17690–17703. https://doi.org/10.1002/jcp.28395
Kalkavan H, Green DR (2018) MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25(1):46–55. https://doi.org/10.1038/cdd.2017.179
Mohamed AA, Khater SI, Hamed Arisha A, Metwally MMM, Mostafa-Hedeab G, El-Shetry ES (2021) Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 768:145288. https://doi.org/10.1016/j.gene.2020.145288
Khazaei S, Ramachandran V, Abdul Hamid R, Mohd Esa N, Etemad A, Moradipoor S, Ismail P (2017) Flower extract of Allium atroviolaceum triggered apoptosis, activated caspase-3 and down-regulated antiapoptotic Bcl-2 gene in HeLa cancer cell line. Biomed Pharmacother 89:1216–1226. https://doi.org/10.1016/j.biopha.2017.02.082
Liu LS, Bai XQ, Gao Y, Wu Q, Ren Z, Li Q, Pan LH, He NY, Peng J, Tang ZH (2017) PCSK9 promotes oxLDL-induced PC12 cell apoptosis through the Bcl-2/Bax-Caspase 9/3 signaling pathway. J Alzheimers Dis 57(3):723–734. https://doi.org/10.3233/jad-161136
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Sci 281(5381):1322–1326. https://doi.org/10.1126/science.281.5381.1322
Xu YR, Yang WX (2018) Roles of three Es-Caspases during spermatogenesis and Cadmium-induced apoptosis in Eriocheir sinensis. Aging 10(5):1146–1165. https://doi.org/10.18632/aging.101454
Sergio L, Thomé AMC, Trajano L, Mencalha AL, Fonseca ASda, Fde Paoli (2018) Photobiomodulation prevents DNA fragmentation of alveolar epithelial cells and alters the mRNA levels of caspase 3 and Bcl-2 genes in acute lung injury. Photochem Photobiol Sci 17(7):975–983. https://doi.org/10.1039/c8pp00109j
Kei S, Sachiko Y-H, Yasuo T, Kimio A, Makoto U (2022) Golgi stress induces upregulation of the ER-Golgi SNARE Syntaxin-5, altered βAPP processing, and Caspase-3-dependent apoptosis in NG108-15 cells. Mol Cell Neurosci 121:103754. https://doi.org/10.1016/j.mcn.2022.103754
Mommersteeg MC, Simovic I, Yu B, van Nieuwenburg SAV, Bruno IMJ, Doukas M, Kuipers EJ, Spaander MCW, Peppelenbosch MP, Castaño-Rodríguez N, Fuhler GM (2022) Autophagy mediates ER stress and inflammation in Helicobacter pylori-related gastric cancer. Gut Microbes 14(1):2015238. https://doi.org/10.1080/19490976.2021.2015238
Murase D, Kusaka-Kikushima A, Hachiya A, Fullenkamp R, Stepp A, Imai A (2020) Autophagy declines with premature skin aging resulting in dynamic alterations in skin pigmentation and epidermal differentiation. Int J Mol Sci 21(16):5708. https://doi.org/10.3390/ijms21165708
Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, Guy CS, Zakharenko SS, Green DR (2019) LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178(3):536–551. https://doi.org/10.1016/j.cell.2019.05.056
Koenig U, Robenek H, Barresi C, Brandstetter M, Resch GP, Gröger M, Pap T, Hartmann C (2020) Cell death induced autophagy contributes to terminal differentiation of skin and skin appendages. Autophagy 16(5):932–945. https://doi.org/10.1080/15548627.2019.1646552
Fernández ÁF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, He C, Ting T, Liu Y, Chiang WC, Marciano DK, Schiattarella GG, Bhagat G, Moe OW, Hu MC, Levine B (2018) Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558(7708):136–140. https://doi.org/10.1038/s41586-018-0162-7
Zhang X, Wang RY (2020) Effects of high-load exercise induced skeletal muscle injury on autophagy ultrastructure and Beclin1 and LC3-II / I in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 36(4):296–300. https://doi.org/10.12047/j.cjap.5944.2020.064
Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E (2021) Empagliflozin attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in high fat diet fed ApoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci 22(2):818. https://doi.org/10.3390/ijms22020818
Satyavarapu EM, Das R, Mandal C, Mukhopadhyay A, Mandal C (2018) Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis 9(10):934. https://doi.org/10.1038/s41419-018-0989-8
Menk M, Graw JA, Poyraz D, Möbius N, Spies CD, Cvon Haefen (2018) von Haefen C Chronic alcohol consumption inhibits autophagy and promotes apoptosis in the liver. Int J Med Sci 15(7):682–688. https://doi.org/10.7150/ijms.25393
Funding
This work was co-financed by the Guangxi Science Natural Foundation for Young Scientists (No. 2019GXNSFBA245045) and the Young and Middle-Aged Backbone Talents Scientific Research Projects of the Affiliated Hospital of Youjiang Medical University for Nationalities in 2021 (No. Y20212606).
Author information
Authors and Affiliations
Contributions
All authors made contributions to the research design. Xi Wei and Dong Li established the animal model and analyzed the samples. Yueling Luo performed a statistical analysis of the data. Biaoliang Wu directed the design of the research. Xi Wei drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Li, D., Luo, Y. et al. Role of Autophagy and Apoptosis in Aluminum Exposure-Induced Liver Injury in Rats. Biol Trace Elem Res 201, 3971–3980 (2023). https://doi.org/10.1007/s12011-022-03497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03497-9