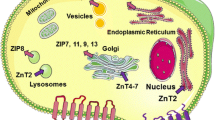
Abstract
Zn status has been related to various chronic diseases presenting oxidative stress and inflammation, such as type 2 diabetes. Zn supplementation has been suggested to be a potential coadjuvant in the management of this condition. Zn transporters constitute a key component in the maintenance of Zn homeostasis. Our aim was to evaluate the modulatory effect of additional Zn (10 or 100 µM; as a ZnSO4*7H20) on the mRNA relative expression of selected Zn transporters (ZnT1, ZnT5, ZnT7, ZIP6, ZIP7, ZIP10, ZIP14), in myoblast (C2C12) cells cultured in normal (10 mM) and high glucose (30 mM), and in the absence or presence of insulin (1 nM), and interleukin-6 (IL-6; 5 nM) for 24 h. The main findings of our study were that in high glucose conditions in absence of insulin or IL-6, additional Zn increased ZnT1 and ZIP6, and decreased ZnT5 and ZIP7 expressions. However, this situation is modified by insulin, where incremental Zn induced increased expressions of ZnT1, ZnT5, and all the ZIP transporters studied. In high glucose conditions and in the presence of IL-6, additional Zn caused increased expressions of ZnT7, ZIP7, and ZIP14, compared with results in the absence of IL-6. This study provides preliminary evidence for the differential expression of selected Zn transporters in C2C12 cells subjected to high glucose and incremental Zn, suggesting that important changes in intracellular Zn distribution take place in response to inflammatory and high-insulin environments. Further study is necessary to understand the implications of these findings.




Similar content being viewed by others
References
Prasad AS, Halsted JA, Nadimi M (1961) Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med 31(4):532–546. https://doi.org/10.1016/0002-9343(61)90137-1
King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ (2015) Biomarkers of nutrition for development (BOND)-zinc review. J Nutr 146(4):858S-885S. https://doi.org/10.3945/jn.115.220079
Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-fer K, Valencia A, Vasquez K, Galgani J, Perez A, Lopez G, Arredondo M, Perez-Bravo F (2013) Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr Bull 34(2):215–521. https://doi.org/10.1177/156482651303400210
Miller J, McLachlan AD, Klug A (1985) Repetitive Zn-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J 4:1609–1614
Krishna SS, Majumdar I, Grishin NV (2003) Structural classification of Zn fingers: survey and summary. Nucleic Acids Res 31:532–550. https://doi.org/10.1093/nar/gkg161
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4:82–91
Kelleher SL, McCormick NH, Velasquez V, Lopez V (2011) Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr 2:101–111. https://doi.org/10.3945/an.110.000232
Kambe T, Tsuji T, Hashimoto A, Itsumura N (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 95:749–784. https://doi.org/10.1152/physrev.00035
Baltaci AK, Yuce K (2018) Zn transporter proteins. Neurochem Res 43(3):517–530. https://doi.org/10.1007/s11064-017-2454-y
Hojyo S, Fukada T (2016) Zn transporters and signaling in physiology and pathogenesis. Arch Biochem Biophys 611:43–50. https://doi.org/10.1016/j.abb.2016.06.020
Kury S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, Moisan J (2002) Identification of SLC39A4 a gene involved in acrodermatitis enteropathica. Nat Genet. 31(3):239–40. https://doi.org/10.1038/ng913
Ruz M, Carrasco F, Rojas P, Basfi-fer K, Hernández MC, Pérez A. (2019) Nutritional effects of zinc on metabolic syndrome and type 2 diabetes: mechanisms and main findings in human studies, BTER. https://doi.org/10.1007/s12011-018-1611-8
Pompano L, Boy E (2021) Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv Nutr. 12(1):141–160. https://doi.org/10.1093/advances/nmaa087
Wang X, Wu W, Zheng W, Fang X, Chen L, Rink L, Min J, Wang F (2019) Zinc supplementation improves glycemic control for diabetes prevention and management a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 110(1):76–90. https://doi.org/10.1093/ajcn/nqz041
Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M (2006) In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119(Pt 20):4199–4206. https://doi.org/10.1242/jcs.03164
Kawasaki E (2012) ZnT8 and type 1 diabetes. Endocr J 59(7):531–537. https://doi.org/10.1507/endocrj.ej12-0069
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB (2010) Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallization and secretion. Diabetologia 53:1656–1668. https://doi.org/10.1007/s00125-010-1733-9
Sladek R, Rochelau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445(7130):881–885. https://doi.org/10.1038/nature05616]
Haase H, Maret W (2005) Fluctuations of cellular, available Zn modulate insulin signaling via inhibition of protein tyrosine phosphatases. J Trace Elem Med Biol 19(1):37–42. https://doi.org/10.1016/j.jtemb.2005.02.004
Moniz T, Amorim MJR, Ferreira R, Nunes A, Silva A, Queirós C, Leite A, Gameiro P, Sarmento B, Remião F, Yoshikawa Y, Sakurai H, Rangel M (2011) Investigation of the insulin-like properties of Zn(II) complexes of 3-hydrox- y-4-pyridinones: identification of a compound with glucose lowering effect in STZ-induced type. J Inorg Biochem 105(12):1675–1682. https://doi.org/10.1016/j.jinorgbio.2011.09.005
Tang XH, Shay NF (2001) Zn has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr 131(5):1414–1420. https://doi.org/10.1093/jn/131.5.1414
Vardatsikos G, Pandey NR, Srivastava AK (2013) Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem 120:8–17. https://doi.org/10.1016/j.jinorgbio.2012.11.006
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Pfaffl M (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res 29:2002–2007
Oteiza PI, Clegg MS, Zago MP, Keen CL (2000) Zn deficiency induces oxidative stress and AP-1 activation in 3T3 cells. Free Rad Biol Med 28(7):1091–1099. https://doi.org/10.1016/s0891-5849(00)00200-8
Ho E, Ames BN (2002) Low intracellular Zn induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. PNAS USA 99(26):16770–16775. https://doi.org/10.1073/pnas.222679399
Ho E, Courtemanche C, Ames BN (2003) Zn deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr 133(8):2543–2548. https://doi.org/10.1093/jn/133.8.2543
Yan M, Song Y, Wong CP, Hardin K, Ho E (2008) Zn deficiency alters DNA damage response genes in normal human prostate epithelial cells. J Nutr 138(4):667–673. https://doi.org/10.1093/jn/138.4.667
Zago MP, Mackenzie GC, Adamo AM, Keen CL, Oteiza PI (2005) Differential modulation of MAP kinases by Zn deficiency in IMR-32 cells: role of H(2)O(2). Antioxid Redox Signaling 7(11–12):1773–1782. https://doi.org/10.1089/ars.2005.7.1773
Wong CP, Ho E (2012) Zn and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res 56:77–87. https://doi.org/10.1002/mnfr.201100511
Lawrence T (2009) The nuclear factor NF-kappa B pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. https://doi.org/10.1101/cshperspect.a001651
Andrews-Guzmán M, Ruz M, Arredondo-Olguin M (2020) Zinc modulates the response to apoptosis in an in vitro model with high glucose and inflammatory stimuli in C2C12 cells. Biol Trace Elem Res Aug 25. https://doi.org/10.1007/s12011-020-02348-9
Ferdowsky PV, Ahuja KDK, Beckett JM, Myers S (2022) Capsaicin and zinc promote glucose uptake in C2C12 skeletal muscle cells through a common calcium signalling pathway. Int J Mol Sci 23:2207. https://doi.org/10.3390/ijms23042207
Contreras-Ferrat A, Lavandero S, Jaimovich E, Klip A (2014) Calcium signaling in insulin action on striad muscle. Cell Calcium 56:390–396. https://doi.org/10.1016/j.ceca.2014.08.012]
Bouron A, Oberwinkler J (2014) Contribution of calcium-conducting channels to the transport of zinc ions. Pflugers Arch Mar. 466(3):381–7. https://doi.org/10.1007/s00424-013-1295-z
Foster M, Samman S (2012) Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4(7):676–694. https://doi.org/10.3390/nu4070676]
Luizzi J, Lichten LA, Rivera S, Cousins RJ (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. PNAS 102:6843–6848. https://doi.org/10.1073/pnas.0502257102]
Aydemir T, Chang S-M, Guthrie GJ, Maki AB, Ryu M-S, Karabiyik A, Cousisn RJ (2012) Zinc transporter ZIP14 functions in hepatic zinc, iron, and glucose homeostasis during the innate immune response (endotoxemia). PLoS ONE 7(10):e48679. https://doi.org/10.1371/journal.pone.0048679
Myers SA, Nield A, Chew GS, Myers MA (2013) The Zn transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS ONE 8:e79316. https://doi.org/10.1371/journal.pone.0079316
Huang L, Kirschke CP, Lay YA, Levy LB, Lamirande DE, Zhang PH (2012) Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J Biol Chem 287:33883–33896. https://doi.org/10.1074/jbc.M111.309666
Paskavitz AL, Quintana J, Cangussu D, Tavera-Montañez C, Xiao Y, Ortiz-Miranda S, Navea JG, Padilla-Benavides T (2018) Differential expression of zinc transporters accompanies the differentiation of C2C12 myoblasts. JTEMB 49:27–34. https://doi.org/10.1016/j.jtemb.2018.04.024
Liu Y, Batchuluun B, Ho L, Zhu D, Prentice KJ, Bhattacharjee A, Zhang M, Pourasgari F, Hardy AB, Taylor KM, Gaisano H, Dai FF, Wheeler MB (2015) Characterization of zinc influx transporters (ZIPs) in pancreatic β cells: roles in regulating cytosolic zinc homeostasis and insulin secretion. J Biol Chem 290(30):18757–18769. https://doi.org/10.1074/jbc.M115.640524
Maxel T, Smidt K, Petersen CC, Honoré B, Christensen AK, Jeppesen PB, Brock B, Rungby J, Palmfeldt J, Larsen A (2019) The zinc transporter Zip14 (SLC39a14) affects beta-cell function: proteomics, gene expression, and insulin secretion studies in INS-1E cells. Sci Rep 9(1):8589. https://doi.org/10.1038/s41598-019-44954-1
Maxel T, Smidt K, Larsen A, Bennetzen M, Cullberg K, Fjeldborg K, Lund S, Pedersen SB, Rungby J (2015) Gene expression of the zinc transporter ZIP14 (SLC39a14) is affected by weight loss and metabolic status and associates with PPARγ in human adipose tissue and 3T3-L1 pre-adipocytes. BMC Obes 2:46. https://doi.org/10.1186/s40608-015-0076-y
Aydemir TB, Troche C, Kim MH, Cousins RJ (2016) Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J Biol Chem 291(46):23939–23951. https://doi.org/10.1074/jbc.M116.748632
Tepaamorndech S, Kirschk CP, Pedersen TL, Keyes WR, Newman JW, Huang L (2016) Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake. FEBS J. 283(2):378–394. https://doi.org/10.1111/febs.13582
Funding
This research was funded by the National Fund for Science and Technological Development (FONDECYT) Chile, grant number 1120323.
Author information
Authors and Affiliations
Contributions
MR, MAG, and MAO: conception and design of research. MAG: performed experiments. MAG and MAO: analyzed data. MAG, MR, and MAO: interpreted results of experiments. MAG and MAO: prepared figures. MAG: drafted manuscript. MAG, MR, and MAO: edited and revised manuscript. MAO and MR: approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Manuel Ruz and Miguel Arredondo received stipends from the research project FONDECYT 1120323. The rest of the authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruz, M., Andrews-Guzmán, M. & Arredondo-Olguín, M. Modulation of Zinc Transporter Expressions by Additional Zinc in C2C12 Cells Cultured in a High Glucose Environment and in the Presence of Insulin or Interleukin-6. Biol Trace Elem Res 201, 3428–3437 (2023). https://doi.org/10.1007/s12011-022-03443-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03443-9