Abstract
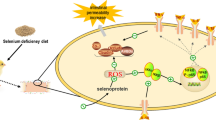
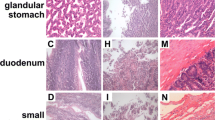
Selenium deficiency can affect the level of selenoprotein in organs and tissues and cause inflammation. However, the mechanism of selenium deficiency on jejunal injury in chickens remains unclear. In this study, we established a selenium deficiency model in chickens by feeding a low selenium diet and observed ultrastructural and pathological changes in the jejunum. The expression levels of 25 selenoproteins, the levels of oxidative stress, tight junction (TJ) proteins, and antimicrobial peptides (AMP), as well as the expression levels of factors related to inflammatory signaling pathways, were examined in the intestine and analyzed using principal component analysis (PCA). The results of PCA and quantitative real-time PCR (qRT-PCR) showed that selenium deficiency mainly affected the expression of antioxidant selenoproteins in chicken jejunum, especially glutathione peroxidases, thioredoxin reductase, and iodothyronine deiodinase, thus weakening the antioxidant function in the intestine and inducing oxidative stress. We also found disruption of intestinal TJ structures, a significant reduction in TJ protein expression, and downregulation of antimicrobial peptide levels, suggesting that selenium deficiency led to damage of the intestinal barrier. In addition, a significant increase in inflammatory cell infiltration and expression of inflammatory factors was observed in the jejunum, indicating that selenium deficiency induces inflammatory injury. In conclusion, selenium deficiency downregulates antioxidant selenoproteins levels, induces oxidative stress, decreases intestinal AMP levels, and leads to inflammatory injury and disruption of the intestinal barrier in the jejunum. These results shed new light on the molecular mechanisms of intestinal damage caused by selenium deficiency.







Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
References
Shi Y et al (2021) Keshan disease: a potentially fatal endemic cardiomyopathy in remote mountains of China. Front Pediatr 9:576916. https://doi.org/10.3389/fped.2021.576916
Shimada BK, Alfulaij N, Seale LA (2021) The impact of selenium deficiency on cardiovascular function. Int J Mol Sci 22(19):10713. https://doi.org/10.3390/ijms221910713
Wang L et al (2020) Serious selenium deficiency in the serum of patients with Kashin-Beck disease and the effect of nano-selenium on their chondrocytes. Biol Trace Elem Res 194(1):96–104. https://doi.org/10.1007/s12011-019-01759-7
Hu X et al (2020) Selenium-mediated gga-miR-29a-3p regulates LMH cell proliferation, invasion, and migration by targeting COL4A2. Metallomics 12(3):449–459. https://doi.org/10.1039/c9mt00266a
Papp LV, Holmgren A, Khanna KK (2010) Selenium and selenoproteins in health and disease. Antioxid Redox Signal 12(7):793–795. https://doi.org/10.1089/ars.2009.2973
Sun Z et al (2018) Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics 10(5):759–767. https://doi.org/10.1039/c8mt00039e
Li J et al (2022) Selenium deficiency induced apoptosis via mitochondrial pathway caused by oxidative stress in porcine gastric tissues. Res Vet Sci 144:142–148. https://doi.org/10.1016/j.rvsc.2021.10.017
Miao Z et al (2022) The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotoxicol Environ Saf 231:113176. https://doi.org/10.1016/j.ecoenv.2022.113176
Bai Y et al (2022) Selenium deficiency causes inflammatory injury in the bursa of Fabricius of broiler chickens by activating the toll-like receptor signaling pathway. Biol Trace Elem Res 200(2):780–789. https://doi.org/10.1007/s12011-021-02688-0
Li S et al (2020) Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics 12(10):1576–1584. https://doi.org/10.1039/d0mt00165a
Tang C et al (2020) Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol 36:101519. https://doi.org/10.1016/j.redox.2020.101519
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):705–743. https://doi.org/10.1089/ars.2011.4145
Maciel-Dominguez A et al (2013) Selenium alters miRNA profile in an intestinal cell line: evidence that miR-185 regulates expression of GPX2 and SEPSH2. Mol Nutr Food Res 57(12):2195–2205. https://doi.org/10.1002/mnfr.201300168
Li M, Cheng W, Zhang L (2021) Maternal selenium deficiency suppresses proliferation, induces autophagy dysfunction and apoptosis in the placenta of mice. Metallomics 13(11):mfab058. https://doi.org/10.1093/mtomcs/mfab058
Li S et al (2021) Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis. J Anim Sci Biotechnol 12(1):65. https://doi.org/10.1186/s40104-021-00587-x
He X et al (2020) Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology, SIgA secretion, and GSH-Px activity. Biol Trace Elem Res 197(2):660–666. https://doi.org/10.1007/s12011-019-02017-6
Zheng Y et al (2021) Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. Biofactors 47(5):788–800. https://doi.org/10.1002/biof.1762
Zhang Y et al (2020) Selenium deficiency induces inflammation via the iNOS/NF-κB pathway in the brain of pigs. Biol Trace Elem Res 196(1):103–109. https://doi.org/10.1007/s12011-019-01908-y
Siomek A (2012) NF-κB signaling pathway and free radical impact. Acta Biochim Pol 59(3):323–331
Wei C et al (2014) NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic Res 48(3):282–291. https://doi.org/10.3109/10715762.2013.865839
Wang J et al (2018) Selenium deficiency induces duodenal villi cell apoptosis via an oxidative stress-induced mitochondrial apoptosis pathway and an inflammatory signaling-induced death receptor pathway. Metallomics 10(10):1390–1400. https://doi.org/10.1039/c8mt00142a
Sun Q et al (2021) Sodium Butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediators Inflamm 2021:6259381. https://doi.org/10.1155/2021/6259381
Emami NK et al (2019) Necrotic enteritis in broiler chickens: the role of tight junctions and mucosal immune responses in alleviating the effect of the disease. Microorganisms 7(8):231. https://doi.org/10.3390/microorganisms7080231
Prasad S et al (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85(9):1139–1162. https://doi.org/10.1038/labinvest.3700316
Oshima, T, Miwa H and Joh T (2008) Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol 23 (2):S146–150.https://doi.org/10.1111/j.1440-1746.2008.05405.x
Poritz LS et al (2007) Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140(1):12–19. https://doi.org/10.1016/j.jss.2006.07.050
Wu Y et al (2019) The role of autophagy in maintaining intestinal mucosal barrier. J Cell Physiol 234(11):19406–19419. https://doi.org/10.1002/jcp.28722
Ayabe T et al (2004) The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol 12(8):394–398. https://doi.org/10.1016/j.tim.2004.06.007
Kuhn KA, Pedraza I, Demoruelle MK (2014) Mucosal immune responses to microbiota in the development of autoimmune disease. Rheum Dis Clin North Am 40(4):711–725. https://doi.org/10.1016/j.rdc.2014.07.013
Cai J et al (2019) Selenium deficiency inhibits myocardial development and differentiation by targeting the mir-215–5p/CTCF axis in chicken. Metallomics 11(2):415–428. https://doi.org/10.1039/c8mt00319j
Chen D et al (2022) Cadmium exposure causes mitochondrial fission and fusion disorder in the pig hypothalamus via the PI3K/AKT pathway. Ecotoxicol Environ Saf 242:113880. https://doi.org/10.1016/j.ecoenv.2022.113880
Lin P et al (2022) Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ROS/TGF-β1/Smad. Toxicology 480:153338. https://doi.org/10.1016/j.tox.2022.153338
He Y et al (2022) Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 307(Pt 1):135662. https://doi.org/10.1016/j.chemosphere.2022.135662
Xu S et al (2021) Pig lung fibrosis is active in the subacute CdCl(2) exposure model and exerts cumulative toxicity through the M1/M2 imbalance. Ecotoxicol Environ Saf 225:112757. https://doi.org/10.1016/j.ecoenv.2021.112757
Miao Z et al (2022) Chlorpyrifos triggers epithelioma papulosum cyprini cell pyroptosis via miR-124–3p/CAPN1 axis. J Hazard Mater 424(Pt A):127318. https://doi.org/10.1016/j.jhazmat.2021.127318
Zheng Y et al (2021) Calcium overload and reactive oxygen species accumulation induced by selenium deficiency promote autophagy in swine small intestine. Anim Nutr 7(4):997–1008. https://doi.org/10.1016/j.aninu.2021.05.005
Liu Q et al (2022) Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol Life Sci 79(2):106. https://doi.org/10.1007/s00018-022-04155-y
Wang Q et al (2021) Low-Se diet can affect sperm quality and testicular glutathione peroxidase-4 activity in rats. Biol Trace Elem Res 199(10):3752–3758. https://doi.org/10.1007/s12011-020-02515-y
Kipp AP et al (2012) Marginal selenium deficiency down-regulates inflammation-related genes in splenic leukocytes of the mouse. J Nutr Biochem 23(9):1170–1177. https://doi.org/10.1016/j.jnutbio.2011.06.011
Sun W et al (2020) Selenium supplementation protects against oxidative stress-induced cardiomyocyte cell cycle arrest through activation of PI3K/AKT. Metallomics 12(12):1965–1978. https://doi.org/10.1039/d0mt00225a
Fan RF et al (2020) Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult Sci 99(11):5430–5439. https://doi.org/10.1016/j.psj.2020.08.031
Oh H, Ghosh S (2013) NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev 252(1):41–51. https://doi.org/10.1111/imr.12033
Moldoveanu B et al (2009) Inflammatory mechanisms in the lung. J Inflamm Res 2:1–11
Gao XJ et al (2019) Selenium deficiency induced an inflammatory response by the HSP60 - TLR2-MAPKs signalling pathway in the liver of carp. Fish Shellfish Immunol 87:688–694. https://doi.org/10.1016/j.fsi.2019.02.017
Du Q et al (2016) Selenium deficiency influences the expression of selenoproteins and inflammatory cytokines in chicken aorta vessels. Biol Trace Elem Res 173(2):501–513. https://doi.org/10.1007/s12011-016-0676-5
Li E, Ajuwon KM (2021) Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. Faseb j 35(2):e21356. https://doi.org/10.1096/fj.202002098R
Li E, Ajuwon KM (2021) Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. FASEB J 35(2):e21356. https://doi.org/10.1096/fj.202002098R
Tang LP et al (2021) Heat stress aggravates intestinal inflammation through TLR4-NF-κB signaling pathway in Ma chickens infected with Escherichia coli O157:H7. Poult Sci 100(5):101030. https://doi.org/10.1016/j.psj.2021.101030
Luo C et al (2020) Betulinic acid ameliorates the T-2 toxin-triggered intestinal impairment in mice by inhibiting inflammation and mucosal barrier dysfunction through the NF-κB signaling pathway. Toxins (Basel) 12(12):794. https://doi.org/10.3390/toxins12120794
Daneshmand A et al (2020) Effects of cLFchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis. Sci Rep 10(1):17704. https://doi.org/10.1038/s41598-020-74754-x
Yoshimura Y (2015) Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult Sci 94(4):804–809. https://doi.org/10.3382/ps/peu021
Zhang H et al (2015) Cathelicidin-BF, a novel antimicrobial peptide from Bungarus fasciatus, attenuates disease in a dextran sulfate sodium model of colitis. Mol Pharm 12(5):1648–1661. https://doi.org/10.1021/acs.molpharmaceut.5b00069
Garcia JS, Byrd JA, Wong EA (2021) Tissue-, age- and dose-dependent changes in avian β-defensin and LEAP2 mRNA abundance in the intestines of Salmonella Typhimurium challenged broilers. Anim Biotechnol 32(5):637–645. https://doi.org/10.1080/10495398.2020.1738449
Ying L et al (2022) Toll-like receptors signaling pathway of quercetin regulating avian beta-defensin in the ileum of broilers. Front Cell Dev Biol 10:816771. https://doi.org/10.3389/fcell.2022.816771
Acknowledgements
The authors thank the Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment, College of Veterinary Medicine, Northeast Agricultural University, for providing conditions.
Funding
This work was supported by the National Natural Science Foundation of China (grant No. 32072811, 31872437).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experiments, and data generation were performed by He Yujiao. Data collection and analysis were performed by Peng Lin. Data curation was performed by Zhao Xiaochun, Fan Xue, and Tang Xinyu. Project administration was performed by Shi Guangliang and Li Shu. The first draft of the manuscript was written by Peng Lin and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures used in this study were approved by the Institutional Animal Care and Use Committee (SRM-11) of Northeast Agricultural University.
Consent for Publication
All authors have read the manuscript and have agreed to submit the manuscript in its current form for consideration for publication in this journal.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
He Yujiao and Peng Lin contributed equally to this work and should be considered co-first authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Y., Peng, L., Zhao, X. et al. Selenium Deficiency Induces Inflammatory Response and Decreased Antimicrobial Peptide Expression in Chicken Jejunum Through Oxidative Stress. Biol Trace Elem Res 201, 3461–3473 (2023). https://doi.org/10.1007/s12011-022-03442-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03442-w