Abstract
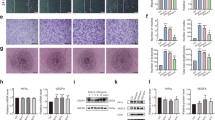
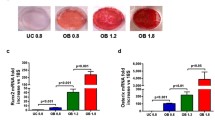
Bone defects are often caused by trauma or surgery and can lead to delayed healing or even bone nonunion, thereby resulting in impaired function of the damaged site. Magnesium ions and related metallic materials play a crucial role in repairing bone defects, but the mechanism remains unclear. In this study, we induced the angiogenic differentiation of bone marrow stromal cells (BMSCs) with different concentrations of magnesium ions. The mechanism was investigated using γ-secretase inhibitor (DAPT) at different time points (7 and 14 days). Angiogenesis, differentiation, migration, and chemotaxis were detected using the tube formation assay, wound-healing assay, and Transwell assay. Besides, we analyzed mRNA expression and the angiogenesis-related protein levels of genes by RT-qPCR and western blot. We discovered that compared with other concentrations, the 5 mM magnesium ion concentration was more conducive to forming tubes. Additionally, hypoxia-inducible factor 1 alpha (Hif-1α) and endothelial nitric oxide (eNOS) expression both increased (p < 0.05). After 7 and 14 days of induction, 5 mM magnesium ion group tube formation, migration, and chemotaxis were enhanced, and the expression of Notch pathway genes increased. Moreover, expression of the Notch target genes hairy and enhancer of split 1 (Hes1) and Hes5 (hairy and enhancer of split 5), as well as the angiogenesis-related genes Hif-1α and eNOS, were enhanced (p < 0.05). However, these trends did not occur when DAPT was applied. This indicates that 5 mM magnesium ion is the optimal concentration for promoting the angiogenesis and differentiation of BMSCs in vitro. By activating the Notch signaling pathway, magnesium ions up-regulate the downstream genes Hes1 and Hes5 and the angiogenesis-related genes Hif-1α and eNOS, thereby promoting the angiogenesis differentiation of BMSCs. Additionally, magnesium ion-induced differentiation enhances the migration and chemotaxis of BMSCs. Thus, we can conclude that magnesium ions and related metallic materials promote angiogenesis to repair bone defects. This provides the rationale for developing artificial magnesium-containing bone materials through tissue engineering.








Similar content being viewed by others
References
Schmitz JP, Hollinger JO (1986) The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res 205:299–308
van de Vijfeijken S, Münker T, Spijker R, Karssemakers L, Vandertop WP, Becking AG, Ubbink DT (2018) Autologous bone is inferior to alloplastic cranioplasties: safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg 117:443–452. https://doi.org/10.1016/j.wneu.2018.05.193
Chen X, Gao CY, Chu XY, Zheng CY, Luan YY, He X, Yang K, Zhang DL (2022) VEGF-loaded heparinised gelatine-hydroxyapatite-tricalcium phosphate scaffold accelerates bone regeneration via enhancing osteogenesis-angiogenesis coupling. Front Bioeng Biotechnol 10:915181. https://doi.org/10.3389/fbioe.2022.915181
Tao ZS, Zhou WS, Tu KK, Huang ZL, Zhou Q, Sun T, Lv YX, Cui W, Yang L (2015) Treatment study of distal femur for parathyroid hormone (1–34) and β-tricalcium phosphate on bone formation in critical-sized defects in osteopenic rats. J Craniomaxillofac Surg 43(10):2136–2143. https://doi.org/10.1016/j.jcms.2015.09.004
Sukegawa S, Kanno T, Matsumoto K, Sukegawa-Takahashi Y, Masui M, Furuki Y (2018) Complications of a poly-L-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology 106(4):360–368. https://doi.org/10.1007/s10266-018-0345-6
Szczęsny G, Kopec M, Politis DJ, Kowalewski ZL, łazarski A, Szolc T 2022 A review on biomaterials for orthopaedic surgery and traumatology: from past to present. Materials (Basel) 15(10) https://doi.org/10.3390/ma15103622
Han H, Loffredo S, Jun I, Edwards J, Kim Y, Seok H, Witte F, Mantovani D, Glyn-Jones S (2019) Current status and outlook on the clinical translation of biodegradable metals. Mater Today 23:57–71. https://doi.org/10.1016/j.mattod.2018.05.018
Romani A (2018) Beneficial role of Mg(2+) in prevention and treatment of hypertension. Int J Hypertens 2018:9013721. https://doi.org/10.1155/2018/9013721
Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L 2017 Biodegradable materials and metallic implants-a review. J Funct Biomater 8(4) https://doi.org/10.3390/jfb8040044
Wang JL, Xu JK, Hopkins C, Chow DH, Qin L (2020) Biodegradable magnesium-based implants in orthopedics-a general review and perspectives. Adv Sci (Weinh) 7(8):1902443. https://doi.org/10.1002/advs.201902443
Bobe K, Willbold E, Haupt M, Reebmann M, Morgenthal I, Andersen O, Studnitzky T, Nellesen J, Tillmann W, Vogt C, Vano-Herrera K, Witte F (2022) Biodegradable open-porous scaffolds made of sintered magnesium W4 and WZ21 short fibres show biocompatibility in vitro and in long-term in vivo evaluation. Acta Biomater. https://doi.org/10.1016/j.actbio.2022.06.005
Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P, Mazzon E, Trubiani O 2020 Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci 21(9) https://doi.org/10.3390/ijms21093242
Maes C (2013) Role and regulation of vascularization processes in endochondral bones. Calcif Tissue Int 92(4):307–323. https://doi.org/10.1007/s00223-012-9689-z
Peng Y, Wu S, Li Y, Crane JL (2020) Type H blood vessels in bone modeling and remodeling. Theranostics 10(1):426–436. https://doi.org/10.7150/thno.34126
Kusumbe AP, Ramasamy SK, Adams RH (2014) Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507(7492):323–328. https://doi.org/10.1038/nature13145
Wang L, Zhou F, Zhang P, Wang H, Qu Z, Jia P, Yao Z, Shen G, Li G, Zhao G, Li J, Mao Y, Xie Z, Xu W, Xu Y, Xu Y (2017) Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis 8(5):e2760. https://doi.org/10.1038/cddis.2017.36
Zhou B, Lin W, Long Y, Yang Y, Zhang H, Wu K, Chu Q (2022) Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther 7(1):95. https://doi.org/10.1038/s41392-022-00934-y
Hall DP, Kovall RA (2019) Structurally conserved binding motifs of transcriptional regulators to notch nuclear effector CSL. Exp Biol Med (Maywood) 244(17):1520–1529. https://doi.org/10.1177/1535370219877818
Díaz-Tocados JM, Herencia C, Martínez-Moreno JM, Montes DOA, Rodríguez-Ortiz ME, Vergara N, Blanco A, Steppan S, Almadén Y, Rodríguez M, Muñoz-Castañeda JR (2017) Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci Rep 7(1):7839. https://doi.org/10.1038/s41598-017-08379-y
Onder S, Calikoglu-Koyuncu AC, Kazmanli K, Urgen M, Kok FN, Torun-Kose G (2018) Magnesium doping on TiN coatings affects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Biomed Mater Eng 29(4):427–438. https://doi.org/10.3233/BME-181000
Caolo V, Molin DG, Post MJ (2012) Notch regulation of hematopoiesis, endothelial precursor cells, and blood vessel formation: orchestrating the vasculature. Stem Cells Int 2012:805602. https://doi.org/10.1155/2012/805602
Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML (2007) Autocrine VEGF signaling is required for vascular homeostasis. Cell 130(4):691–703. https://doi.org/10.1016/j.cell.2007.06.054
Maes C, Clemens TL (2014) Angiogenic-osteogenic coupling: the endothelial perspective. Bonekey Rep 3:578. https://doi.org/10.1038/bonekey.2014.73
Li X, Wang Y, Shi L, Li B, Li J, Wei Z, Lv H, Wu L, Zhang H, Yang B, Xu X, Jiang J (2020) Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology 18(1):113. https://doi.org/10.1186/s12951-020-00670-x
Montazersaheb S, Kabiri F, Saliani N, Nourazarian A, Avci ÇB, Rahbarghazi R, Nozad CH (2018) Prolonged incubation with Metformin decreased angiogenic potential in human bone marrow mesenchymal stem cells. Biomed Pharmacother 108:1328–1337. https://doi.org/10.1016/j.biopha.2018.09.135
Mauffrey C, Barlow BT, Smith W (2015) Management of segmental bone defects. J Am Acad Orthop Surg 23(3):143–153. https://doi.org/10.5435/JAAOS-D-14-00018
Vasyliev RG, Oksymets VM, Rodnichenko AE, Zlatska AV, Gubar OS, Gordiienko IM, Zubov DO (2017) Tissue-engineered bone for treatment of combat related limb injuries. Exp Oncol 39(3):191–196
Xing J, Jin H, Hou T, Chang Z, Luo F, Wang P, Li Z, Xie Z, Xu J (2014) Establishment of a bilateral femoral large segmental bone defect mouse model potentially applicable to basic research in bone tissue engineering. J Surg Res 192(2):454–463. https://doi.org/10.1016/j.jss.2014.05.037
Sader MS, Martins VC, Gomez S, Legeros RZ, Soares GA (2013) Production and in vitro characterization of 3D porous scaffolds made of magnesium carbonate apatite (MCA)/anionic collagen using a biomimetic approach. Mater Sci Eng C Mater Biol Appl 33(7):4188–4196. https://doi.org/10.1016/j.msec.2013.06.006
Wang W, Yeung K (2017) Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater 2(4):224–247. https://doi.org/10.1016/j.bioactmat.2017.05.007
Tarafder S, Dernell WS, Bandyopadhyay A, Bose S (2015) SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: mechanical properties and in vivo osteogenesis in a rabbit model. J Biomed Mater Res B Appl Biomater 103(3):679–690. https://doi.org/10.1002/jbm.b.33239
Ziche M, Morbidelli L (2000) Nitric oxide and angiogenesis.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. https://doi.org/10.1080/14653240600855905
Li Y, Pan Q, Xu J, He X, Li HA, Oldridge DA, Li G, Qin L (2021) Overview of methods for enhancing bone regeneration in distraction osteogenesis: potential roles of biometals. J Orthop Translat 27:110–118. https://doi.org/10.1016/j.jot.2020.11.008
Ohyama T 2019)New aspects of magnesium function: a key regulator in nucleosome self-assembly, chromatin folding and phase separation. Int J Mol Sci 20(17) https://doi.org/10.3390/ijms20174232
Yamanaka R, Shindo Y, Oka K (2019) Magnesium is a key player in neuronal maturation and neuropathology. Int J Mol Sci 20(14) https://doi.org/10.3390/ijms20143439
Zhang X, Zu H, Zhao D, Yang K, Tian S, Yu X, Lu F, Liu B, Yu X, Wang B, Wang W, Huang S, Wang Y, Wang Z, Zhang Z (2017) Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: in vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater 63:369–382. https://doi.org/10.1016/j.actbio.2017.08.051
Feyerabend F, Witte F, Kammal M, Willumeit R (2006) Unphysiologically high magnesium concentrations support chondrocyte proliferation and redifferentiation. Tissue Eng 12(12):3545–3556. https://doi.org/10.1089/ten.2006.12.3545
Cipriano AF, Sallee A, Tayoba M, Cortez AM, Lin A, Guan RG, Zhao ZY, Liu H (2017) Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg-Zn-Sr alloys. Acta Biomater 48:499–520. https://doi.org/10.1016/j.actbio.2016.10.020
Yoshizawa S, Brown A, Barchowsky A, Sfeir C (2014) Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater 10(6):2834–2842. https://doi.org/10.1016/j.actbio.2014.02.002
Zhai W, Lu H, Wu C, Chen L, Lin X, Naoki K, Chen G, Chang J (2013) Stimulatory effects of the ionic products from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater 9(8):8004–8014. https://doi.org/10.1016/j.actbio.2013.04.024
Rankin EB, Giaccia AJ, Schipani E (2011) A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Curr Osteoporos Rep 9(2):46–52. https://doi.org/10.1007/s11914-011-0047-2
Deng Z, Lin B, Jiang Z, Huang W, Li J, Zeng X, Wang H, Wang D, Zhang Y (2019) Hypoxia-mimicking cobalt-doped borosilicate bioactive glass scaffolds with enhanced angiogenic and osteogenic capacity for bone regeneration. Int J Biol Sci 15(6):1113–1124. https://doi.org/10.7150/ijbs.32358
Siebel C, Lendahl U (2017) Notch signaling in development, tissue homeostasis, and disease. Physiol Rev 97(4):1235–1294. https://doi.org/10.1152/physrev.00005.2017
Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalén M, Gerhardt H, Betsholtz C (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445(7129):776–780. https://doi.org/10.1038/nature05571
Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104(9):3219–3224. https://doi.org/10.1073/pnas.0611206104
Suchting S, Freitas C, le Noble F, Benedito R, Bréant C, Duarte A, Eichmann A (2007) The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA 104(9):3225–3230. https://doi.org/10.1073/pnas.0611177104
Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Ylä-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K (2008) Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454(7204):656–660. https://doi.org/10.1038/nature07083
Luo Z, Shang X, Zhang H, Wang G, Massey PA, Barton SR, Kevil CG, Dong Y (2019) Notch signaling in osteogenesis, osteoclastogenesis, and angiogenesis. Am J Pathol 189(8):1495–1500. https://doi.org/10.1016/j.ajpath.2019.05.005
Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444(7122):1083–1087. https://doi.org/10.1038/nature05313
Ramasamy SK, Kusumbe AP, Wang L, Adams RH (2014) Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507(7492):376–380. https://doi.org/10.1038/nature13146
Ramasamy SK, Kusumbe AP, Schiller M, Zeuschner D, Bixel MG, Milia C, Gamrekelashvili J, Limbourg A, Medvinsky A, Santoro MM, Limbourg FP, Adams RH (2016) Blood flow controls bone vascular function and osteogenesis. Nat Commun 7:13601. https://doi.org/10.1038/ncomms13601
Semenova D, Bogdanova M, Kostina A, Golovkin A, Kostareva A, Malashicheva A (2020) Dose-dependent mechanism of Notch action in promoting osteogenic differentiation of mesenchymal stem cells. Cell Tissue Res 379(1):169–179. https://doi.org/10.1007/s00441-019-03130-7
Yang M, Li CJ, Sun X, Guo Q, Xiao Y, Su T, Tu ML, Peng H, Lu Q, Liu Q, He HB, Jiang TJ, Lei MX, Wan M, Cao X, Luo XH (2017) MiR-497∼195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1α activity. Nat Commun 8:16003. https://doi.org/10.1038/ncomms16003
Guo CL (2022) Self-sustained regulation or self-perpetuating dysregulation: ROS-dependent HIF-YAP-Notch signaling as a double-edged sword on stem cell physiology and tumorigenesis. Front Cell Dev Biol 10:862791. https://doi.org/10.3389/fcell.2022.862791
De Francesco EM, Maggiolini M, Musti AM 2018 Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int J Mol Sci 19(7) https://doi.org/10.3390/ijms19072011
Nathan J, Ramachandran A (2022) Efficacy of marine biomolecules on angiogenesis by targeting hypoxia inducible factor/vascular endothelial growth factor signaling in zebrafish model. J Biochem Mol Toxicol 36(2):e22954. https://doi.org/10.1002/jbt.22954
Niu H, Gao N, Dang Y, Guan Y, Guan J (2022) Delivery of VEGF and delta-like 4 to synergistically regenerate capillaries and arterioles in ischemic limbs. Acta Biomater 143:295–309. https://doi.org/10.1016/j.actbio.2022.03.021
Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T (2017) The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 20(3):291–302. https://doi.org/10.1007/s10456-017-9541-1
Lee HW, Shin JH, Simons M (2022) Flow goes forward and cells step backward: endothelial migration. Exp Mol Med 54(6):711–719. https://doi.org/10.1038/s12276-022-00785-1
Etienne-Manneville S (2013) Microtubules in cell migration. Annu Rev Cell Dev Biol 29:471–499. https://doi.org/10.1146/annurev-cellbio-101011-155711
Michaelis UR (2014) Mechanisms of endothelial cell migration. Cell Mol Life Sci 71(21):4131–4148. https://doi.org/10.1007/s00018-014-1678-0
Hou CY, Ma CY, Yuh CH (2022) WNK1 kinase signaling in metastasis and angiogenesis. Cell Signal 96:110371. https://doi.org/10.1016/j.cellsig.2022.110371
Li M, Wu Y, Ye L 2022 The role of amino acids in endothelial biology and function. Cells-Basel 11(8) https://doi.org/10.3390/cells11081372
Chen J, Jiang L, Yu XH, Hu M, Zhang YK, Liu X, He P, Ouyang X (2021) Endocan: a key player of cardiovascular disease. Front Cardiovasc Med 8:798699. https://doi.org/10.3389/fcvm.2021.798699
Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM (2014) Osteoblast recruitment routes in human cancellous bone remodeling. Am J Pathol 184(3):778–789. https://doi.org/10.1016/j.ajpath.2013.11.022
Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19(2):329–344. https://doi.org/10.1016/j.devcel.2010.07.010
Zhang ZZ, Zhou YF, Li WP, Jiang C, Chen Z, Luo H, Song B (2019) Local administration of magnesium promotes meniscal healing through homing of endogenous stem cells: a proof-of-concept study. Am J Sports Med 47(4):954–967. https://doi.org/10.1177/0363546518820076
Barkefors I, Le Jan S, Jakobsson L, Hejll E, Carlson G, Johansson H, Jarvius J, Park JW, Li JN, Kreuger J (2008) Endothelial cell migration in stable gradients of vascular endothelial growth factor A and fibroblast growth factor 2: effects on chemotaxis and chemokinesis. J Biol Chem 283(20):13905–13912. https://doi.org/10.1074/jbc.M704917200
Caiado F, Real C, Carvalho T, Dias S (2008) Notch pathway modulation on bone marrow-derived vascular precursor cells regulates their angiogenic and wound healing potential. PLoS ONE 3(11):e3752. https://doi.org/10.1371/journal.pone.0003752
Koyanagi M, Bushoven P, Iwasaki M, Urbich C, Zeiher AM, Dimmeler S (2007) Notch signaling contributes to the expression of cardiac markers in human circulating progenitor cells. Circ Res 101(11):1139–1145. https://doi.org/10.1161/CIRCRESAHA.107.151381
Li H, Yu B, Zhang Y, Pan Z, Xu W, Li H (2006) Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem Biophys Res Commun 341(2):320–325. https://doi.org/10.1016/j.bbrc.2005.12.182
Xu LL, Fu HX, Zhang JM, Feng FE, Wang QM, Zhu XL, Xue J, Wang CC, Chen Q, Liu X, Wang YZ, Qin YZ, Kong Y, Chang YJ, Xu LP, Liu KY, Huang XJ, Zhang XH (2017) Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the Notch-1/Jagged-1 signaling pathway. Stem Cells Dev 26(22):1648–1661. https://doi.org/10.1089/scd.2017.0078
Hu N, Zou L (2022) Multiple functions of Hes genes in the proliferation and differentiation of neural stem cells. Ann Anat 239:151848. https://doi.org/10.1016/j.aanat.2021.151848
Funding
This research was continuously funded by National Natural Science Foundation of China (No. 82172432; 82102568 and 82001319), National & Local Joint Engineering Research Center of Orthopaedic Biomaterials (XMHT20190204007), Shenzhen High-level Hospital Construction Fund, Shenzhen Key Medical Discipline Construction Fund (No. SZXK023), Shenzhen “San-Ming” Project of Medicine (No. SZSM201612092), Research and Development Projects of Shenzhen (No. Z2021N054), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515012586), Bethune Charitable Foundation and CSPC Osteoporosis Research Foundation Project (No. G-X-2020–1107-21), and The Scientific Research Foundation of PEKING UNIVERSITY SHENZHEN HOSPITAL (No. KYQD2021099).
Author information
Authors and Affiliations
Contributions
Conceptualization, H.Z. and F.Y.; methodology, T.Q. and Y.Z.; software, W.Z. and G.L.; formal analysis, J.W. and B.Z.; investigation, H.Q., J.W., and B.Z.; data curation, Y.Z., Y.C., and W.Z.; writing—original draft preparation, H.Q., J.W., and B.Z.; writing—review and editing, F.Y. and H.Z.; supervision, F.Y. and H.Z.; funding acquisition, F.Y. and H.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, H., Weng, J., Zhou, B. et al. Magnesium Ions Promote In Vitro Rat Bone Marrow Stromal Cell Angiogenesis Through Notch Signaling. Biol Trace Elem Res 201, 2823–2842 (2023). https://doi.org/10.1007/s12011-022-03364-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03364-7