Abstract
Background
Lead occupational exposure is now a main concern in the modern world. Lead is a non-biodegradable element with multi-devastating effects on different organs. Acute or chronic exposure to lead is reported to be one of the most important causes of infertility both in males and females basically by inducing oxidative stress and apoptosis.
Objectives
The current study scrutinized the mitigating effects of N-acetylcysteine (NAC) on lead toxicity, oxidative stress, and apoptotic/anti-apoptotic genes in the testis tissues of male rats.
Methods
Rats were randomly divided into a control group (G1) and four study groups treated with single and continuous doses of lead with and without NAC administration. Malondialdehyde (MDA), total antioxidant capacity (TAC), and 8-hydroxy-2'-deoxyguanosine (8-OHdG) were analyzed as oxidative stress biomarkers and the expression of apoptosis-related genes was studied using RT-PCR.
Results
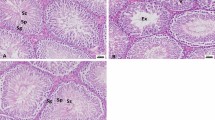
Continuous exposure to lead caused a significant decrease in sperm count, motility, viability, and morphology (P < 0.001). Number of germinal cells, Leydig cells, spermatocytes, and the diameter of seminiferous tubule were significantly decreased (P < 0.001) in G3 group. Continuous exposure to lead significantly decreased TAC content, but increased the levels of MDA and 8-OHdG (P < 0.001). Administration of continuous dose of lead dramatically increased expression of Bax, Caspase-3, Caspase-8, Cytochrome-C, MMP2, and MMP9 genes in testicular tissue. NAC treatments not only improved morphological changes and sperm quality, but also enhanced antioxidant balance and modulated apoptosis process in testicular tissue of rats.
Conclusion
Lead exposure strongly motivated testicular cells towards apoptosis, caused an oxidant/antioxidant imbalance, and decreased sperm quality along with morphological changes in testis cells. NAC treatments was associated with protective effects on testicular tissue mainly by rebalancing of the antioxidants capacity, as well as downregulation of apoptosis-related genes.


Similar content being viewed by others
References
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164
Alquezar C, Felix JB, McCandlish E, Buckley BT, Caparros-Lefebvre D, Karch CM, Golbe LI, Kao AW (2020) Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons. Sci Rep 10:569
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972
Nigra AE, Ruiz-Hernandez A, Redon J, Navas-Acien A, Tellez-Plaza M (2016) Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Environ Health Rep 3:416–433
Zhang S, Sun L, Zhang J, Liu S, Han J, Liu Y (2020) Adverse impact of heavy metals on bone cells and bone metabolism dependently and independently through anemia. Adv Sci (Weinh) 7:2000383
Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA et al (2016) Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 44:275–278
Benoff S, JacobI A, Hurley R (2000) Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update 6:107–121
Colagar AH, Marzony ET, Chaichi MJ (2009) Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res 29:82–88
Li C, Zhao K, Zhang H, Liu L, Xiong F, Wang K, Chen B (2018) Lead exposure reduces sperm quality and DNA integrity in mice. Environ Toxicol 33:594–602
Adhikari N, Sinha N, Narayan R, Saxena DK (2001) Lead-induced cell death in testes of young rats. J Appl Toxicol 21:275–277
Mirnamniha M, Faroughi F, Tahmasbpour E, EbrahimiA P, Harchegani B (2019) An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health 34:339–348
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Marzouni ET, HarcheganiI AB, Layali (2021) Chromosomal aneuploidies and associated rare genetic syndromes involved in male infertility. J Men’s Health 17:7–17
Colagar AHMET (2009) Ascorbic acid in human seminal plasma: determination and its relationship to sperm quality. J Clin Biochem Nutr 45:144–149
Khamisabadi A, Tahmasbpour E, Ghanei MSA (2020) Roles of matrix metalloproteinases (MMPs) in SM-induced pathologies. Toxin Reviews 39:24–33
Rana SV (2008) Metals and apoptosis: recent developments. J Trace Elem Med Biol 22:262–284
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
BaSalamah MA, Abdelghany AH, El-Boshy M, Ahmad J, Idris S, Refaat B (2018) Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep 8:1–13
Flora SJ, Saxena G, Mehta A (2007) Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+. J Pharmacol Exp Ther 322:108–116
Morales ME, Derbes RS, Ade CM, Ortego JC, Stark J, Deininger PL, Roy-Engel AM (2016) Heavy metal exposure influences double strand break DNA repair outcomes. PLoS ONE 11:e0151367
Zeisel SH (2004) Antioxidants suppress apoptosis. J Nutr 134:3179S-3180S
Schwalfenberg GK (2021) N-Acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J Nutr Metab 9949453
Kelly GS (1998) Clinical applications of N-acetylcysteine. Altern Med Rev 3:114–127
Luczak MWAZ (2013) Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium(VI), cadmium(II), and cobalt(II). Free Radic Biol Med 65:262–269
Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A (2017) A review on various uses of N-acetyl cysteine. Cell J 19:11–17
Yedjou CG, Waters D, Tchounwou PB (2008) N-Acetyl-cysteine protection against lead-induced oxidative stress and genotoxicity in human liver carcinoma (HepG(2)) cells. Metal Ions Biol Med 10:419–424
Rahmani Talatappeh N, RanjiA N, Harchegani B (2021) The effect of N-acetyl cysteine on oxidative stress and apoptosis in the liver tissue of rats exposed to cadmium. Arch Environ Occup Health 76:518–525
Ciftci H, Verit A, Savas M, Yeni E, Erel O (2009) Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology 74:73–76
Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH (2019) Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrinol 17:1–9
Alizadeh B, Salehzadeh A, Ranji N, Arasteh A (2022) Effects of N-acetyl cysteine on genes expression of c-myc, and Ask-1, histopathological, oxidative stress, inflammation, and apoptosis in the liver of male rats exposed to cadmium. Biol Trace Elem Res 200:661–668
Jaafarzadeh, M., R. Mahjoob Khaligh, Z. Mohsenifar, A. Shabani, M. Rezvani Gilkalaei, S. Rajabi KeleshteriA. Beigi Harchegani (2021) Protecting effects of N-acetyl cysteine supplementation against lead and cadmium-induced brain toxicity in rat models. Biol Trace Elem Res.
Ma Z, Chu L, Liu H, Wang W, Li J, Yao W, Yi J, Gao Y (2017) Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci Rep 7:44819
Benzie IF (1996) Lipid peroxidation: a review of causes, consequences, measurement and dietary influences. Int J Food Sci Nutr 47:233–261
Rao B, Soufir JC, Martin M, David G (1989) Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res 24:127–134
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Almansour MI (2009) Histological alterations induced by lead in the testes of the quail Coturnix coturnix. Res J Environ Toxicol 3:24–30
Offor SJ, Mbagwu HO, Orisakwe OE (2019) Improvement of lead acetate-induced testicular injury and sperm quality deterioration by Solanum anomalum Thonn. ex. schumach fruit extracts in albino rats. J Family Reprod Health 13:98–108
Elgawish RAR, Abdelrazek HMA (2014) Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicol Rep 1:795–801
Dribben WH, Creeley CE, Farber N (2013) Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol 33:473–480
Yedjou CG, Tchounwou CK, Haile S, Edwards F, Tchounwou PB (2010) N-acetyl-cysteine protects against DNA damage associated with lead toxicity in HepG2 cells. Ethn Dis 20: S1–101–3.
AnnabiBerrahal A, Nehdi A, Hajjaji N, Gharbi N, El-Fazâa S (2007) Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol 330:581–588
Navabpour S, Yamchi A, Bagherikia S, Kafi H (2020) Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol Mol Biol Plants 26:793–802
Kumar R, Reddy AG, Anjaneyulu Y, Reddy GD (2010) Oxidative stress induced by lead and antioxidant potential of certain adaptogens in poultry. Toxicol Int 17:45–48
Shraideh Z, Badran Z, Hunaiti A, Battah A (2018) Association between occupational lead exposure and plasma levels of selected oxidative stress related parameters in Jordanian automobile workers. Int J Occup Med Environ Health 31:517–525
Li-Hui Xu, Fang-Fang Mu, Zhao J-H, He Q, Yang C-L (2015) Lead induces apoptosis and histone hyperacetylation in rat cardiovascular tissues. PLoS ONE 10:e0129091
Wang J, Li M, Zhang W, Gu A, Dong J, Li J et al (2018) Protective effect of N-acetylcysteine against oxidative stress induced by zearalenone via mitochondrial apoptosis pathway in SIEC02 cells. Toxins (Basel) 10:407
Cay A, Alver A, Küçük M, Işik O, Eminağaoğlu MS, Karahan SC, Değer O (2006) The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res 131:199–203
Kazaz IO, Demir S, Yulug E, Colak F, Bodur A, Yaman SO, Karaguzel E, Mentese A (2019) N-acetylcysteine protects testicular tissue against ischemia/reperfusion injury via inhibiting endoplasmic reticulum stress and apoptosis. J Pediatr Urol 15:253.e1-253.e8
Malmir M, SoleimaniMehranjani M, NaderiNoreini S, Faraji T (2018) Protective antioxidant effects of N-acetylcysteine against impairment of spermatogenesis caused by paranonylphenol. Andrologia 50:e13114
Shieh P, Jan CR, Liang WZ (2019) The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology 417:1–14
Kumar BA, Reddy AG, Kumar PR, Reddy YR, Rao TM, Haritha C (2013) Protective role of N-Acetyl L-Cysteine against reproductive toxicity due to interaction of lead and cadmium in male Wistar rats. J Nat Sci Biol Med 4:414–419
Chen S, Ren Q, Zhang J, Ye Y, Zhang Z, Xu Y et al (2014) N-acetyl-L-cysteine protects against cadmium-induced neuronal apoptosis by inhibiting ROS-dependent activation of Akt/mTOR pathway in mouse brain. Neuropathol Appl Neurobiol 40:759–777
Acknowledgements
This work is part of PhD thesis which is performed at Islamic Azad University, Rasht, Iran (162373285). We would like to express our special thanks of gratitude to all the staff and members who helped us through this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedini Bajgiran, F., Khazaei Koohpar, Z. & Salehzadeh, A. Effects of N-Acetylcysteine Supplementation on Oxidative Stress and Expression of Apoptosis-Related Genes in Testicular Tissue of Rats Exposed to Lead. Biol Trace Elem Res 201, 2407–2415 (2023). https://doi.org/10.1007/s12011-022-03325-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03325-0