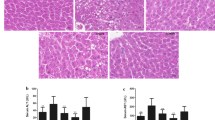
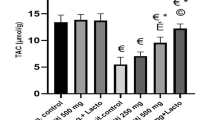
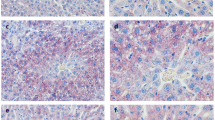
Abstract
Current study was aimed to investigate the ability of L.acidophilus SNZ 86 to biotransform inorganic selenium to a more active organic form, resulting in trace element enrichment. Selenium-enriched L. acidophilus SNZ 86 has been shown to be effective in the treatment of a variety of gastrointestinal illnesses, indicating the need for additional research to determine the full potential of this therapeutic strategy in the treatment of metabolic disorders. Herein, we employed the western style diet-induced model of non-alcoholic fatty liver disease (NAFLD) to explore the therapeutic effect of selenium-enriched probiotic (SP). Male Sprague Dawley rats (160–180 g) were fed a high-fat (58% Kcal of fat) and high-fructose (30% w/v) diet for 12 weeks to develop an animal model mimicking NAFLD. High-fat and High-fructose diet-fed rats exhibited hyperglycemia, hyperlipidemia, insulin resistance, abnormal liver function test, increased hepatic oxidative stress, and steatosis. SP was then administered orally (L acidophilus 1 × 109 CFU/ml containing 0.4 g Se/day; p.o.) for 8 weeks. The selenium enrichment within L. acidophilus SNZ 86 was validated by TEM, which allowed for visualisation of the selenium deposition and size distribution in the probiotic. In NAFLD control rats, the expression of autophagy proteins (LC-3 A/B and Beclin), AMPK, and SIRT-1 was significantly reduced indicating downregulation of autophagy. However, supplementation of SP ameliorates hepatic steatosis as evidenced by improved biochemical markers and autophagic activation via upregulation of the AMPK and SIRT-1 pathway showing the relevance of autophagy in the disease aetiology. Collectively, these findings provide us with a better understanding of the role of SP in the treatment of hepatic steatosis and establish a therapeutic basis for potential clinical application of SP in the prevention of NAFLD and associated pathological conditions.
Graphical abstract







Similar content being viewed by others
Data Availability
All data and materials support their published claims and comply with field standards data will be available on demand.
References
Friedman SL et al (2018) Mechanisms of NAFLD development and therapeutic strategies. Nat Med 24(7):908–922. https://doi.org/10.1038/s41591-018-0104-9
Levene AP, Goldin RD (2012) The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 61(2):141–152. https://doi.org/10.1111/j.1365-2559.2011.04145.x
Chalsani N et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1):328–357
Michelotti GA, Machado MV, Diehl AM (2013) NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 10(11):656–665. https://doi.org/10.1038/nrgastro.2013.183
Hardy T et al (2016) Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol 11:451–496. https://doi.org/10.1146/annurev-pathol-012615-044224
Ji YX et al (2018) The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med 24(2):213–223. https://doi.org/10.1038/nm.4461
Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12 Suppl 2(2): p. 1542–52. https://doi.org/10.1038/sj.cdd.4401765
Czaja MJ (2016) Function of autophagy in nonalcoholic fatty liver disease. Dig Dis Sci 61(5):1304–1313. https://doi.org/10.1007/s10620-015-4025-x
Martinez-Lopez N, Singh R (2015) Autophagy and lipid droplets in the liver. Annu Rev Nutr 35:215–237. https://doi.org/10.1146/annurev-nutr-071813-105336
Cantó C et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458(7241):1056–1060. https://doi.org/10.1038/nature07813
Guo Y et al (2017) Targeting Sirt1 in a rat model of high-fat diet-induced non-alcoholic fatty liver disease: comparison of Gegen Qinlian decoction and resveratrol. Exp Ther Med 14(5):4279–4287. https://doi.org/10.3892/etm.2017.5076
Mariani S et al (2015) Plasma levels of SIRT1 associate with non-alcoholic fatty liver disease in obese patients. Endocrine 49(3):711–716. https://doi.org/10.1007/s12020-014-0465-x
Purushotham A et al (2009) Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9(4):327–338. https://doi.org/10.1016/j.cmet.2009.02.006
Pfluger PT et al (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 105(28):9793–9798. https://doi.org/10.1073/pnas.0802917105
Banerjee J, Bruckbauer A, Zemel MB (2016) Activation of the AMPK/Sirt1 pathway by a leucine–metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 65(11):1679–1691. https://doi.org/10.1016/j.metabol.2016.06.011
Heo J et al (2016) Gut microbiota modulated by probiotics and Garcinia cambogia extract correlate with weight gain and adipocyte sizes in high fat-fed mice. Sci Rep 6:33566. https://doi.org/10.1038/srep33566
Naito E et al (2011) Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol 110(3):650–657. https://doi.org/10.1111/j.1365-2672.2010.04922.x
Park SS et al (2018) Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J Endocrinol 237(2):87–100. https://doi.org/10.1530/JOE-17-0592
Wendt S et al (2019) Selenium in Cardiac Surgery. Nutr Clin Pract 34(4):528–539. https://doi.org/10.1002/ncp.10326
Schäfer K et al (2004) Effects of selenium deficiency on fatty acid metabolism in rats fed fish oil-enriched diets. J Trace Elem Med Biol 18(1):89–97
Stapleton SR et al (1997) Selenium: potent stimulator of tyrosyl phosphorylation and activator of MAP kinase. Biochim Biophys Acta 1355(3):259–269. https://doi.org/10.1016/s0167-4889(96)00140-1
Xu C et al (2018) Biogenic synthesis of novel functionalized selenium nanoparticles by Lactobacillus casei ATCC 393 and its protective effects on intestinal barrier dysfunction caused by enterotoxigenic Escherichia coli K88. 9: p. 1129.
Nido SA et al (2016) Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. 171(2): p. 399–409.
Ibrahim HA et al (2012) Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. 147(1): p. 251-260.
Liu Y et al (2015) Protective effects of Selenium-enriched probiotics on carbon tetrachloride-induced liver fibrosis in rats. J Agric Food Chem 63(1):242–249. https://doi.org/10.1021/jf5039184
Graham L, Orenstein JMJNp (2007) Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. 2(10): p. 2439–2450.
Srinivasan K et al (2005) Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 52(4):313–320. https://doi.org/10.1016/j.phrs.2005.05.004
Wada T et al (2013) Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. Am J Physiol Endocrinol Metab 305(11):E1415–E1425. https://doi.org/10.1152/ajpendo.00419.2013
Ohkawa H, Ohishi N, Yagi KJAb (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Rahman I, Kode A, Biswas SKJNp (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1(6):3159–3165. https://doi.org/10.1038/nprot.2006.378
Amara VR, Surapaneni SK, Tikoo K (2017) Dysregulation of microRNAs and renin-angiotensin system in high salt diet-induced cardiac dysfunction in uninephrectomized rats. PLoS ONE 12(7):e0180490. https://doi.org/10.1371/journal.pone.0180490
Karpe PA, Tikoo K (2014) Heat shock prevents insulin resistance–induced vascular complications by augmenting angiotensin-(1–7) signaling. Diabetes 63(3):1124–1139. https://doi.org/10.2337/db13-1267
Shen XH et al (2005) Effects of dietary supplementation with vitamin E and selenium on rat hepatic stellate cell apoptosis. World J Gastroenterol 11(32):4957–4961. https://doi.org/10.3748/wjg.v11.i32.4957
Ding M et al (2010) Selenium supplementation decreases hepatic fibrosis in mice after chronic carbon tetrachloride administration. Biol Trace Elem Res 133(1):83–97. https://doi.org/10.1007/s12011-009-8414-x
Zhao Z et al (2020) Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol 104(12):5273–5282. https://doi.org/10.1007/s00253-020-10633-9
Nagy G et al (2016) In situ electron microscopy of lactomicroselenium particles in probiotic bacteria. Int J Mol Sci 17(7):1047
Krausova G et al (2020) Development of selenized lactic acid bacteria and their selenium bioaccummulation capacity. Fermentation 6(3):91
Krausova G et al (2021) In vivo bioavailability of selenium in selenium-enriched Streptococcus thermophilus and Enterococcus faecium in CD IGS rats. Antioxidants 10(3):463
Khambu B et al (2018) Autophagy in non-alcoholic fatty liver disease and alcoholic liver disease. Liver Res 2(3):112–119. https://doi.org/10.1016/j.livres.2018.09.004
Tanaka S et al (2016) Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 64(6):1994–2014. https://doi.org/10.1002/hep.28820
Song GL et al (2018) Selenium-enriched yeast inhibited beta-amyloid production and modulated autophagy in a triple transgenic mouse model of Alzheimer’s disease. Metallomics 10(8):1107–1115. https://doi.org/10.1039/c8mt00041g
Song YM et al (2015) Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy 11(1):46–59. https://doi.org/10.4161/15548627.2014.984271
Sun Y et al (2018) Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br J Pharmacol 175(2):374–387. https://doi.org/10.1111/bph.14079
Funding
This study is funded by the National Institute of Pharmaceutical Education and Research.
Author information
Authors and Affiliations
Contributions
RP and KT designed the research work and wrote the manuscript; RP, NS, SKW, and SS performed the experiments and analysed and compiled the data; KT supervised and edited the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All institutional and national guidelines for the care and use of laboratory animals were followed. The animal studies were approved by the animal ethics committee of the NIPER S.A.S Nagar (IAEC18/09).
Consent for Publication
All authors are willing to publish their research work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pant, R., Sharma, N., Kabeer, S.W. et al. Selenium-Enriched Probiotic Alleviates Western Diet-Induced Non-alcoholic Fatty Liver Disease in Rats via Modulation of Autophagy Through AMPK/SIRT-1 Pathway. Biol Trace Elem Res 201, 1344–1357 (2023). https://doi.org/10.1007/s12011-022-03247-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03247-x