Abstract
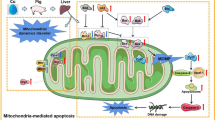
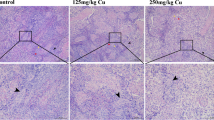
Copper (Cu) is an essential trace element for growth and development in most organisms. However, environmental exposure to high doses of Cu can damage multiple organs. To investigate the underlying mechanism of Cu toxicity on mitochondrial dynamics and mitophagy in the cerebrum of pigs, 60 30-day-old pigs were randomly divided into three groups and treated with different contents of anhydrous Cu sulfate in the diets (Cu 10 mg/kg, control group; Cu 125 mg/kg, group I; Cu 250 mg/kg, group II) for 80 days. The Cu levels and histological changes in the cerebrum were measured. Moreover, the protein and mRNA expression levels related to mitophagy and mitochondrial dynamics were determined. The results showed that the contents of Cu were increased in the cerebrum with increasing dietary Cu. Vacuolar degeneration was found in group I and group II compared to the control group. Additionally, the protein and mRNA expression levels of PINK1, Parkin, and Drp1 and the protein level of LC3-II were remarkably upregulated with increasing levels of dietary Cu. Nevertheless, the protein and mRNA expression levels of MFN1 and MFN2 and the mRNA expression of P62 were obviously downregulated in a Cu dose-dependent manner. Overall, these results suggested that excess Cu could trigger mitochondrial dynamics disorder and mitophagy in the pig cerebrum, which provided a novel insight into Cu-induced toxicology.







Similar content being viewed by others
References
Denoyer D, Clatworthy S, Cater MA (2018) Copper complexes in cancer therapy. Met Ions Life Sci 18. https://doi.org/10.1515/9783110470734-022
Sullivan B, Robison G, Osborn J, Kay M, Thompson P, Davis K, Zakharova T, Antipova O, Pushkar Y (2017) On the nature of the Cu-rich aggregates in brain astrocytes. Redox Biol 11:231–239. https://doi.org/10.1016/j.redox.2016.12.007
Jiang X, Xiong Z, Liu H, Liu G, Liu W (2017) Distribution, source identification, and ecological risk assessment of heavy metals in wetland soils of a river–reservoir system. Environ Sci Pollut R 24(1):436–444. https://doi.org/10.1007/s11356-016-7775-x
Xu D, Fu R, Liu H, Guo X (2021) Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: a critical review. J Clean Prod 286:124989. https://doi.org/10.1016/j.jclepro.2020.124989
Lutsenko S (2016) Copper trafficking to the secretory pathway. Metallomics 8(9):84–852. https://doi.org/10.1039/c6mt00176a
Nie X, Wang Y, Zhao H, Guo M, Liu Y, Xing M (2020) As3+ or/and Cu2+ exposure triggers oxidative stress imbalance, induces inflammatory response and apoptosis in chicken brain. Ecotox Environ Safe 203:110993. https://doi.org/10.1016/j.ecoenv.2020.110993
Czlonkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML (2018) Wilson disease. Nat Rev Dis Primers 4(1):21. https://doi.org/10.1038/s41572-018-0018-3
Schlief ML, Gitlin JD (2006) Copper homeostasis in the CNS: a novel link between the NMDA receptor and copper homeostasis in the hippocampus. Mol Neurobiol 33(2):81–90. https://doi.org/10.1385/MN:33:2:81
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3):151–166. https://doi.org/10.1016/j.tins.2017.01.002
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13. https://doi.org/10.1042/BJ20081386
Garza-Lombo C, Pappa A, Panayiotidis MI, Franco R (2020) Redox homeostasis, oxidative stress and mitophagy. Mitochondrion 51:105–117. https://doi.org/10.1016/j.mito.2020.01.002
Lazarou M, Jin SM, Kane LA, Youle RJ (2012) Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell 22(2):320–333. https://doi.org/10.1016/j.devcel.2011.12.014
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. https://doi.org/10.1038/cdd.2012.81
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183(5):795–803. https://doi.org/10.1083/jcb.200809125
Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39(1):503–536. https://doi.org/10.1146/annurev.genet.38.072902.093019
Bertholet AM, Delerue T, Millet AM, Moulis MF, David C, Daloyau M, Arnaune-Pelloquin L, Davezac N, Mils V, Miquel MC, Rojo M, Belenguer P (2016) Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis 90:3–19. https://doi.org/10.1016/j.nbd.2015.10.011
Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM (2009) Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187(7):959–966. https://doi.org/10.1083/jcb.200906083
Rovira-Llopis S, Banuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM (2017) Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 11:637–645. https://doi.org/10.1016/j.redox.2017.01.013
Chen Y, Dorn GN (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340(6131):471–475. https://doi.org/10.1126/science.1231031
Borchard S, Bork F, Rieder T, Eberhagen C, Popper B, Lichtmannegger J, Schmitt S, Adamski J, Klingenspor M, Weiss KH, Zischka H (2018) The exceptional sensitivity of brain mitochondria to copper. Toxicol In Vitro 51:11–22. https://doi.org/10.1016/j.tiv.2018.04.012
Liao Z, Cao H, Dai X, Xing C, Xu X, Nie G, Zhang C (2018) Molybdenum and cadmium exposure influences the concentration of trace elements in the digestive organs of Shaoxing duck (Anas platyrhyncha). Ecotoxicol Environ Saf 164:75–83. https://doi.org/10.1016/j.ecoenv.2018.07.119
Chen H, Liu G, Qiao N, Kang Z, Hu L, Liao J, Yang F, Pang C, Liu B, Zeng Q, Li Y, Li Y (2020) Toxic effects of arsenic trioxide on spermatogonia are associated with oxidative stress, mitochondrial dysfunction, autophagy and metabolomic alterations. Ecotoxicol Environ Saf 190:110063. https://doi.org/10.1016/j.ecoenv.2019.110063
Li Q, Liao J, Lei C, Shi J, Zhang H, Han Q, Guo J, Hu L, Li Y, Pan J, Tang Z (2021) Metabolomics analysis reveals the effect of copper on autophagy in myocardia of pigs. Ecotoxicol Environ Saf 213:112040. https://doi.org/10.1016/j.ecoenv.2021.112040
Roubicek DA, Souza-Pinto NC (2017) Mitochondria and mitochondrial DNA as relevant targets for environmental contaminants. Toxicology 391:100–108. https://doi.org/10.1016/j.tox.2017.06.012
Zheng W, Monnot AD (2012) Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol Therapeut 133(2):177–188. https://doi.org/10.1016/j.pharmthera.2011.10.006
Hung YH, Bush AI, Cherny RA (2010) Copper in the brain and Alzheimer’s disease. J Biol Inorg Chem 15(1):61–76. https://doi.org/10.1007/s00775-009-0600-y
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40(2):280–293. https://doi.org/10.1016/j.molcel.2010.09.023
Magrane J, Sahawneh MA, Przedborski S, Estevez AG, Manfredi G (2012) Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci 32(1):229–242. https://doi.org/10.1523/JNEUROSCI.1233-11.2012
Choi B, Zheng W (2009) Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res 1248:14–21. https://doi.org/10.1016/j.brainres.2008.10.056
Yang F, Liao J, Pei R, Yu W, Han Q, Li Y, Guo J, Hu L, Pan J, Tang Z (2018) Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 204:36–43. https://doi.org/10.1016/j.chemosphere.2018.03.192
Zhao Y, Li HX, Luo Y, Cui JG, Talukder M, Li JL (2022) Lycopene mitigates DEHP-induced hepatic mitochondrial quality control disorder via regulating SIRT1/PINK1/mitophagy axis and mitochondrial unfolded protein response. Environ Pollut 292(Pt B):118390. https://doi.org/10.1016/j.envpol.2021.118390
Pickrell AM, Youle RJ (2015) The roles of PINK1, Parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85(2):257–273. https://doi.org/10.1016/j.neuron.2014.12.007
Okatsu K, Uno M, Koyano F, Go E, Kimura M, Oka T, Tanaka K, Matsuda N (2013) A dimeric PINK1-containing complex on depolarized mitochondria stimulates Parkin recruitment. J Biol Chem 288(51):36372–36384. https://doi.org/10.1074/jbc.M113.509653
Yu S, Du M, Yin A, Mai Z, Wang Y, Zhao M, Wang X, Chen T (2020) Bcl-xL inhibits PINK1/Parkin-dependent mitophagy by preventing mitochondrial Parkin accumulation. Int J Biochem Cell Biol 122:105720. https://doi.org/10.1016/j.biocel.2020.105720
Liao J, Li Q, Hu Z, Yu W, Zhang K, Ma F, Han Q, Zhang H, Guo J, Hu L, Pan J, Li Y, Tang Z (2021) Mitochondrial miR-1285 regulates copper-induced mitochondrial dysfunction and mitophagy by impairing IDH2 in pig jejunal epithelial cells. J Hazard Mater 422:126899. https://doi.org/10.1016/j.jhazmat.2021.126899
Chuang K, Chang C, Chang S, Huang S, Chuang S, Li Z, Wang S, Kao J, Chen Y, Shieh J (2020) Imiquimod-induced ROS production disrupts the balance of mitochondrial dynamics and increases mitophagy in skin cancer cells. J Dermatol Sci 98(3):152–162. https://doi.org/10.1016/j.jdermsci.2020.03.009
Westermann B (2012) Bioenergetic role of mitochondrial fusion and fission. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1817(10):1833–1838. https://doi.org/10.1016/j.bbabio.2012.02.033
Mishra P, Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Bio 15(10):634–646. https://doi.org/10.1038/nrm3877
Xiaoyu, Wang Huabin, Cao Yukun, Fang He, Bai Jing, Chen Chenghong, Xing Yu, Zhuang Xiaoquan, Guo Guoliang, Hu Fan, Yang (2022) Activation of endoplasmic reticulum-mitochondria coupling drives copper-induced autophagy in duck renal tubular epithelial cells. Ecotoxicology and Environmental Safety 235113438. https://doi.org/10.1016/j.ecoenv.2022.113438
Acknowledgements
We thank Shuzhou Wang for helping in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 32072930 and 31572585) and the National Key R & D Program of China (Nos. 2016YFD0501205 and 2017YFD0502200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinrun Li, Yuman Bai, and Haihua Huo share the first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Bai, Y., Huo, H. et al. Long-term Copper Exposure Induces Mitochondrial Dynamics Disorder and Mitophagy in the Cerebrum of Pigs. Biol Trace Elem Res 201, 1197–1204 (2023). https://doi.org/10.1007/s12011-022-03224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03224-4