Abstract
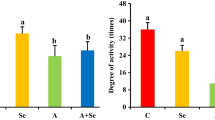
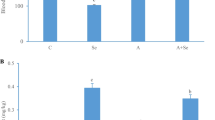
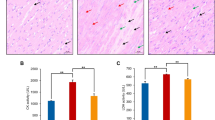
L-Selenomethionine is one of the important organic selenium sources. The supplementation of L-selenomethionine in diets is significant to improve the health of pigs. Ammonia is a major pollutant in the atmosphere and piggery, posing a threat to human and animal health. Although ammonia exposure can damage the heart, the mechanism of cardiac toxicity by ammonia is still unknown. In this study, we investigated the mechanism of cardiac injury induced by ammonia exposure in pigs and the protective effect of L-selenomethionine on its cardiotoxicity. The results showed that the blood ammonia content of pig increased significantly in ammonia group, the expressions of energy metabolism-related genes (LDHA, PDK4, HK2, and CPTIB) and the oxidative stress indexes were significantly changed (P < 0.05), the AMPK/PPAR-γ/NF-κB signaling pathways were activated, the chromatin edge aggregation and nuclear pyknosis were observed in ultrastructure, the apoptotic cells were significantly increased (P < 0.05), and the mRNA and protein expressions of apoptosis-related genes (Bcl-2, Bax, Cyt-c, caspase-3, and caspase-9) were significantly affected (P < 0.05). The above changes were significantly alleviated in ammonia + L-selenomethionine group, but there were still significant differences compared with the C group (P < 0.05). Our results indicated that ammonia exposure could cause energy metabolism disorder and oxidative stress and induce apoptosis of cardiomyocytes through AMPK/PPAR-γ/NF-κB pathways, which could lead to cardiac injury and affect cardiac function. L-Selenomethionine could effectively alleviate the cardiac damage caused by ammonia and antagonize the cardiotoxicity of ammonia.







Similar content being viewed by others
References
Xing H, Luan S, Sun Y, Sa R, Zhang H (2016) Effects of ammonia exposure on carcass traits and fatty acid composition of broiler meat. Anim Nutr 2:282–287
Ni JQ (2015) Research and demonstration to improve air quality for the U.S. animal feeding operations in the 21st century – a critical review. Environ Pollut 200:105–119
Battye W, Aneja VP, Roelle PA (2003) Evaluation and improvement of ammonia emissions inventories. Atmos Environ 37:3873–3883
Ni JQ, Shi C, Liu S, Richert BT, Vonderohe CE, Radcliffe JS, (2019) Effects of antibiotic-free pig rearing on ammonia emissions from five pairs of swine rooms in a wean-to-finish experiment. Environ Int 131. https://doi.org/10.1016/j.envint.2019.104931
Saraswati Sharma SK, Saxena M, Mandal TK (2019) Characteristics of gaseous and particulate ammonia and their role in the formation of secondary inorganic particulate matter at Delhi. India Atmos Res 218:34–49
Chen Y, Chang ET, Liu Q, Cai Y, Zhang Z, Chen G, Huang QH, Xie SH, Cao SM, Jia WH, Zheng Y, Li Y, Lin L, Ernberg I, Wang D, Chen W, Feng R, Huang G, Zeng YX, Adami HO, Ye W (2021) Occupational exposures and risk of nasopharyngeal carcinoma in a high-risk area: a population-based case-control study. Cancer. https://doi.org/10.1002/cncr.33536
Nieder R, Benbi DK (2021) Reactive nitrogen compounds and their influence on human health: an overview. Rev Environ Health. https://doi.org/10.1515/reveh-2021-0021
Li Z, Miao Z, Ding L, Teng X, Bao J (2021) Energy metabolism disorder mediated ammonia gas-induced autophagy via AMPK/mTOR/ULK1-Beclin1 pathway in chicken livers. Ecotoxicol Environ Saf 217(undefined):112219. https://doi.org/10.1016/j.ecoenv.2021.112219
Duan Y, Xiong D, Wang Y, Li H, Dong Hong B, Zhang J (2021) Toxic effects of ammonia and thermal stress on the intestinal microbiota and transcriptomic and metabolomic responses of Litopenaeus vannamei. Sci Total Environ 754(undefined):141867. https://doi.org/10.1016/j.scitotenv.2020.141867
Wang H, Zeng X, Zhang X, Liu H, Xing H (2021) Ammonia exposure induces oxidative stress and inflammation by destroying the microtubule structures and the balance of solute carriers in the trachea of pigs. Ecotoxicol Environ Saf 212:111974. https://doi.org/10.1016/j.ecoenv.2021.111974
Xia C, Zhang X, Zhang Y, Li J, Xing H (2021) Ammonia exposure causes the disruption of the solute carrier family gene network in pigs. Ecotoxicol Environ Saf 210:111870. https://doi.org/10.1016/j.ecoenv.2020.111870
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90(5):1193–1209
Roy MJ, Vom A, Czabotar PE, Lessene G (2014) Cell death and the mitochondria: therapeutic targeting of the BCL-2 family-driven pathway. Brit J Pharmacol 171:1973–1987
Hardie GD (2003) Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144:5179–5183
Chen L, Liu T, Zhang S, Zhou J, Wang Y, Di W (2014) Succinate dehydrogenase subunit B inhibits the AMPK-HIF-1α pathway in human ovarian cancer in vitro. J Ovarian Res 7:115. https://doi.org/10.1186/s13048-014-0115-1
Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R et al (2011) Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol 122:389–395
Vucetic M, Otasevic V, Korac A, Stancic A, Jankovic A, Markelic M (2011) Interscapular brown adipose tissue metabolic reprogramming during cold acclimation: interplay of HIF-1α and AMPKα. Biochim Biophys Acta 1810:1252–1261
Li H, Satriano J, Thomas JL, Miyamoto S, Sharma K, Pastor-Soler NM, Hallows KR, Singh P (2015) Interactions between HIF-1α and AMPK in the regulation of cellular hypoxia adaptation in chronic kidney disease. Am J Physiol Renal Physiol 309(5):F414–F428. https://doi.org/10.1152/ajprenal.00463.2014
Zhao Y, Zhang WD, Liu XQ, Zhang PF, Hao YN, Li L, Chen L, Shen W, Tang XF, Min LJ, Meng QS, Wang SK, Yi B, Zhang HF (2016) Hydrogen sulfide and/or ammonia reduces spermatozoa motility through AMPK/AKT related pathways. Sci Rep 6:37884–37895
Başalan Över S, Guven C, Taskin E, Çakmak A, Piner Benli P, Sevgiler Y (2021) Effects of different ammonia levels on tribenuron methyl toxicity in Daphnia magna. Arch Environ Contam Toxicol https://doi.org/10.1007/s00244-021-00841-3
Liao HH, Jia XH, Liu HJ, Yang Z, Tang QZ (2017) The role of PPARs in pathological cardiac hypertrophy and heart failure. Curr Pharmaceut Des 23:1677–1686
Zhao H, Wang Y, Liu Y, Yin K, Wang D, Li B, Yu H, Xing M (2021) ROS-induced hepatotoxicity under cypermethrin: involvement of the crosstalk between Nrf2/Keap1 and NF-κB/iκB-α pathways regulated by proteasome. Environ Sci Technol 55:6171–6183
Wang H, Zhang Y, Han Q, Xu Y, Hu G, Xing H (2020) The inflammatory injury of heart caused by ammonia is realized by oxidative stress and abnormal energy metabolism activating inflammatory pathway. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140532
Ostovar M, Akbari A, Anbardar Mohammad H, Iraji A, Salmanpour M, Hafez Ghoran S, Heydari M, Shams M (2020) Effects of Citrullus colocynthis L. in a rat model of diabetic neuropathy. J. Integr. Med. 18(1):59–67
Wang S, Li X, Wang W, Zhang H, Xu S (2019) Application of transcriptome analysis: oxidative stress, inflammation and microtubule activity disorder caused by ammonia exposure may be the primary factors of intestinal microvilli deficiency in chicken. Sci Total Environ 696:134035. https://doi.org/10.1016/j.scitotenv.2019.134035
Moris D, Spartalis M, Spartalis E, Karachaliou GS, Karaolanis GI, Tsourouflis G, Tsilimigras DI, Tzatzaki E, Theocharis S (2017) The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann Transl Med 5(16):326. https://doi.org/10.21037/atm.2017.06.27
Lee Y, Gustafsson AB (2009) Role of apoptosis in cardiovascular disease. Apoptosis 14:536–548
Williams TA, Bonham LA, Bernier NJ (2017) High environmental ammonia exposure has developmental-stage specific and long-term consequences on the cortisol stress response in zebrafish. Gen Comp Endocrinol 254:97–106
Chu JH, Yan YX, Gao PC, Chen XW, Fan RF (2020) Response of selenoproteins gene expression profile to mercuric chloride exposure in chicken kidney. Res Vet Sci 133:4–11
Fan RF, Liu JX, Yan YX, Wang L, Wang ZY (2020) Selenium relieves oxidative stress, inflammation, and apoptosis within spleen of chicken exposed to mercuric chloride. Poult Sci 99:5430–5439
Li S, Tang T, Guo P, Zou Q, Ao X, Hu L, Tan L (2019) A meta-analysis of randomized controlled trials: efficacy of selenium treatment for sepsis. Med (Baltim) 98(9):14733. https://doi.org/10.1097/MD.0000000000014733
Jin X, Xu Z, Zhao X, Chen M, Xu S (2017) The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180:259–266
Feng C, Li D, Chen M, Jiang L, Liu X, Li Q, Geng C, Sun X, Yang G, Zhang L, Yao X (2019) Citreoviridin induces myocardial apoptosis through PPAR-γ-mTORC2-mediated autophagic pathway and the protective effect of thiamine and selenium. Chem Biol Interact 311(undefined):108795. https://doi.org/10.1016/j.cbi.2019.108795
Xue C, Li Y, Lv H, Zhang L, Bi C, Dong N, Shan A, Wang J (2021) Oleanolic acid targets the gut-liver axis to alleviate metabolic disorders and hepatic steatosis. J Agr Food Chem. https://doi.org/10.1021/acs.jafc.1c02257
Di Lorenzo T, Melita M, Cifoni M, Galassi DMP, Iannucci A, Biricolti S, Gori M, Baratti M (2017) Effect of ammonia on the gene expression levels of the freshwater cyclopoid Eucyclops serrulatus. Environ Toxicol Pharmacol 51:138–141
Wang W, Shi Q, Wang S, Zhang H, Xu S (2020) Ammonia regulates chicken tracheal cell necroptosis via the LncRNA-107053293/MiR-148a-3p/FAF1 axis. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121626
Wang Y, Zhao H, Liu Y, Li J, Nie X, Huang P, Xing M (2021) Environmentally relevant concentration of sulfamethoxazole-induced oxidative stress-cascaded damages in the intestine of grass carp and the therapeutic application of exogenous lycopene. Environ Pollut 274:116519. https://doi.org/10.1016/j.envpol.2021.116597
Komoike Y, Matsuoka M (2019) In vitro and in vivo studies of oxidative stress responses against acrylamide toxicity in zebrafish. J Hazard Mater 365:430–439
Hu X, Chi Q, Wang D, Chi X, Teng X, Li S (2018) Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotoxicol Environ Saf 64:201–209
Chi Q, Chi X, Hu X, Wang S, Zhang H, Li S (2018) The effects of atmospheric hydrogen sulfide on peripheral blood lymphocytes of chickens: perspectives on inflammation, oxidative stress and energy metabolism. Environ. Res. 167(undefined):1–6. https://doi.org/10.1016/j.envres.2018.06.051
Zhang M, Li M, Wang R, Qian Y (2018) Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol 79:313–320
Yadu B, Chandrakar V, Korram J, Satnami ML, Kumar M, Keshavkant S (2018) Silver nanoparticle modulates gene expressions, glyoxalase system and oxidative stress markers in fluoride stressed Cajanus cajan L. J Hazard Mater 353:44–52
Satta S, Mahmoud AM, Wilkinson FL, Yvonne Alexander M, White SJ (2017) The role of Nrf2 in cardiovascular function and disease. Oxid Med Cell Longev 2017:9237263. https://doi.org/10.1155/2017/9237263
Zhang F, Fan Y, Zhang D, Chen S, Bai X, Ma X, Xie Z, Xu H (2020) Effect and mechanism of the algicidal bacterium Sulfitobacter porphyrae ZFX1 on the mitigation of harmful algal blooms caused by Prorocentrum donghaiense. Environ Pollut 263. https://doi.org/10.1016/j.envpol.2020.114475
Liu H, Xu H, Huang K (2017) Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 9:21–37
Tsutsui H, Ide T, Shiomi T, Kang D, Hayashidani S, Suematsu N, Wen J, Utsumi H, Hamasaki N, Takeshita A (2001) 8-oxo-dGTPase, which prevents oxidative stress-induced DNA damage, increases in the mitochondria from failing hearts. Circulation 104(24):2883–2885
Ren S, Liu J, Feng Y, Li Z, He L, Li L, Cao X, Wang Z, Zhang Y (2019) Knockdown of circDENND4C inhibits glycolysis, migration and invasion by up-regulating miR-200b/c in breast cancer under hypoxia. J Exp Clin Cancer Res 38(1). https://doi.org/10.1186/s13046-019-1398-2
Wu J, Zhao Y, Park Y, Lee J, Gao L, Zhao J, Wang L (2018) Loss of PDK4 switches the hepatic NF-κB/TNF pathway from pro-survival to pro-apoptosis. Hepatology 68(3):1111–1124
Taggart K, Estrada A, Thompson P, Lourenco F, Kirmani S, Suzuki-Hatano S, Pacak Christina A (2017) PDK4 deficiency induces intrinsic apoptosis in response to starvation in fibroblasts from Doberman pinschers with dilated cardiomyopathy. Biores Open Access 6(1):182–191
Tang S, Xie J, Wu W, Yi B, Liu L, Zhang H (2020) High ammonia exposure regulates lipid metabolism in the pig skeletal muscle via mTOR pathway. Sci Total Environ 740:139917. https://doi.org/10.1016/j.scitotenv.2020.139917
Guo J, Xing H, Chen M, Wang W, Zhang H, Xu S (2019) H2S inhalation-induced energy metabolism disturbance is involved in LPS mediated hepatocyte apoptosis through mitochondrial pathway. Sci Total Environ 663:380–386
Zhao H, Wang Y, Guo M, Liu Y, Yu H, Xing M (2020) Environmentally relevant concentration of cypermethrin or/and sulfamethoxazole induce neurotoxicity of grass carp: involvement of blood-brain barrier, oxidative stress and apoptosis. Sci Total Environ 17:143054. https://doi.org/10.1016/j.scitotenv.2020.143054
Yuan Y, Wang Y, Liu X, Luo B, Zhang L, Zheng F, Li X, Guo L, Wang L, Jiang M, Pan Y, Yan Y, Yang J, Chen S, Wang J, Tang J (2019) KPC1 alleviates hypoxia/reoxygenation-induced apoptosis in rat cardiomyocyte cells though BAX degradation. J Cell Physiol 234(12):22921–22934. https://doi.org/10.1002/jcp.28854
Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A (2002) Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419:634–637
Shah SWA, Chen J, Han Q, Xu Y, Ishfaq M, Teng X (2020) Ammonia inhalation impaired immune function and mitochondrial integrity in the broiler bursa of fabricius: implication of oxidative stress and apoptosis. Ecotoxicol Environ Saf 190:110078. https://doi.org/10.1016/j.ecoenv.2019.110078
Wang S, Song P, Zou M (2012) AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin Sci (Lond) 122(12):555–573. https://doi.org/10.1042/CS20110625
Pan H, Wang H, Zhu L, Wang X, Cong Z, Sun K, Fan Y (2013) The involvement of Nrf2ARE pathway in regulation of apoptosis in human glioblastoma cell U251. Neurol Res 35:71–78
Cabezas-Sanchez P, Rainieri S, Conlledo N, Barranco A, Sanz-Landaluze J, Camara C, Luque-Garcia JL (2019) Impact of selenium co-administration on methylmercury exposed eleutheroembryos and adult zebrafish (Danio rerio): changes in bioaccumulation and gene expression. Chemosphere 236:124295. https://doi.org/10.1016/j.chemosphere.2019.07.026
Marettová E, Maretta M, Legáth J (2015) Toxic effects of cadmium on testis of birds and mammals: a review. Anim. Reprod. Sci. 155(undefined):1–10
Zhang R, Liu Y, Xing L, Zhao N, Zheng Q, Li J, Bao J (2018) The protective role of selenium against cadmium-induced hepatotoxicity in laying hens: expression of Hsps and inflammation-related genes and modulation of elements homeostasis. Ecotoxicol Environ Saf 159:205–212
Kim N-H, Kang PM (2010) Apoptosis in cardiovascular diseases: mechanism and clinical implications. Korean Circ J 40(7):299–305
Lee HY, Naha N, Kim JH, Jo MJ, Min KS, Seong HH, Shin DH, Kim MO (2008) Age- and area-dependent distinct effects of ethanol on Bax and Bcl-2 expression in prenatal rat brain. J Microbiol Biotechnol 18:1590–1598
Funding
The study was supported by the Earmarked Fund for China Agriculture Research System (Project No. CARS-35) and the Natural Science Foundation of Heilongjiang Province (LC2018009). Earmarked Fund for China Agriculture Research System,CARS-35,Houjuan Xing
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, X., Wang, A., Wang, X. et al. Evaluation of L-Selenomethionine on Ameliorating Cardiac Injury Induced by Environmental Ammonia. Biol Trace Elem Res 200, 4712–4725 (2022). https://doi.org/10.1007/s12011-021-03071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03071-9