Abstract
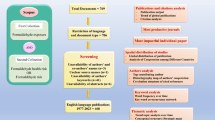
It has been reported that heavy metals have adverse effects on the immune system. However, the relationship between heavy metal exposure and allergic outcomes remains unclear. This systematic review was conducted to examine whether heavy metal exposure is associated with allergic outcomes during childhood. We performed a systematic search of all relevant articles in Web of Science, EMBASE, and PubMed, from inception through to November 2020. We used odds ratio (OR) and the standard mean differences (SMDs) with 95% confidence intervals (CIs) to present estimates from individual studies. In addition, random-effects meta-analysis was used to pool the data. We also conducted the meta-regression and subgroup analysis to explore potential sources of heterogeneity. After duplicate removal, we finally included 35 articles in the systematic review and meta-analysis from an initial 11,181 articles. The overall results showed that copper (Cu) was associated with asthma (pooled SMD = 1.50, 95% CI = 0.13–2.86); in the subgroup analysis, the results indicated that lead (Pb) was associated with asthma (pooled OR = 6.27, 95% CI = 2.24–17.56), and Cu and Pb were connected with atopic dermatitis (SMD = − 1.05, 95% CI = − 1.45 to − 0.65; SMD = 5.68, 95% CI = 5.05–6.32), respectively. Mercury (Hg) was associated with atopic dermatitis (pooled OR = 1.13, 95% CI = 1.04–1.22) and wheeze (OR = 1.20, 95% CI = 1.05–1.37). The meta-analysis results indicate that Cu might be connected with childhood asthma, but not with other allergic diseases; Hg and Pb may have no association with allergic diseases during childhood. Given some limits observed in the current studies, more prospective cohort studies are still needed to verify our findings. Review registration: PROSPERO CRD42020222167.
Graphic Abstract





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
References
Hoskins G, McCowan C, Neville RG, Thomas GE, Smith B, Silverman S (2000) Risk factors and costs associated with an asthma attack. Thorax 55:19–24. https://doi.org/10.1136/thorax.55.1.19
Martinez FD, Vercelli D (2013) Asthma. Lancet 382:1360–1372. https://doi.org/10.1016/s0140-6736(13)61536-6
Reynolds LA, Finlay BB (2017) Early life factors that affect allergy development. Nat Rev Immunol 17:518–528. https://doi.org/10.1038/nri.2017.39
Elias BC, Silva JB, Mais LA, Warkentin S, Konstantyner T, Solé D (2019) Factors associated with asthma in brazilian adolescents: national adolescent school-based health survey (pense-2012). Rev Paul Pediatr 37:406–413. https://doi.org/10.1590/1984-0462/;2019;37;4;00002
To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP (2012) Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 12:204. https://doi.org/10.1186/1471-2458-12-204
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI (2009) Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 124:1251–8.e23. https://doi.org/10.1016/j.jaci.2009.10.009
Wei X, Jiang P, Liu J, Sun R, Zhu L (2020) Association between probiotic supplementation and asthma incidence in infants: a meta-analysis of randomized controlled trials. The Journal of asthma : official journal of the Association for the Care of Asthma 57:167–178. https://doi.org/10.1080/02770903.2018.1561893
Paiva Ferreira LKD, Paiva Ferreira LAM, Monteiro TM, Bezerra GC, Bernardo LR, Piuvezam MR (2019) Combined allergic rhinitis and asthma syndrome (CARAS). Int Immunopharmacol 74:105718. https://doi.org/10.1016/j.intimp.2019.105718
Murrison LB, Brandt EB, Myers JB, Hershey GKK (2019) Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 129:1504–1515. https://doi.org/10.1172/jci124612
Skevaki C, Renz H (2018) Advances in mechanisms of allergic disease in 2017. J Allergy Clin Immunol 142:1730–1739. https://doi.org/10.1016/j.jaci.2018.09.027
Kumar M, Nandi M, Pakshirajan K (2021) Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J Environ Manage 278:111555. https://doi.org/10.1016/j.jenvman.2020.111555
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14. https://doi.org/10.3390/ijerph14121504
Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R (2019) Metal toxicity links to Alzheimer’s disease and neuroinflammation. J Mol Biol 431:1843–1868. https://doi.org/10.1016/j.jmb.2019.01.018
Tan JH, Duan JC (2013) Heavy metals in aerosol in China: pollution, sources, and control strategies. Journal of Graduate University of Chinese Academy of Science 2:145–155
Wang HD, Fang FM, Xie HF (2010) Research Situation and Outlook on Heavy Metal Pollution in Water Environment of China. Guangdong Trace Elements Science 1:14–18
China MO (2014) National Soil Pollution Survey Bulletin 5. 10-11
Wu KG, Chang CY, Yen CY, Lai CC (2019) Associations between environmental heavy metal exposure and childhood asthma: a population-based study. J Microbiol Immunol Infect 52:352–362. https://doi.org/10.1016/j.jmii.2018.08.001
El Sherbeny MM, Behairy OG, Mohammad OI, Elsayed AM (2016) Serum levels of lead and copper in a group of Egyptian children with bronchial asthma. Egyptian Journal of Pediatric Allergy and Immunology 14:47–52
Kim JH, Jeong KS, Ha EH, Park H, Ha M, Hong YC, Lee SJ, Lee KY, Jeong J, Kim Y (2013) Association between prenatal exposure to cadmium and atopic dermatitis in infancy. J Korean Med Sci 28:516–521. https://doi.org/10.3346/jkms.2013.28.4.516
Wei J, Zhang JJ, Ji JS (2019) Association of environmental exposure to heavy metals and eczema in US population: analysis of blood cadmium, lead, and mercury. Arch Environ Occup Health 74:239–251. https://doi.org/10.1080/19338244.2018.1467874
Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, Gregson J, Willeit P, Warnakula S, Khan H, Chowdhury S, Gobin R, Franco OH, Di Angelantonio E (2018) Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310. https://doi.org/10.1136/bmj.k3310
Warthon-Medina M, Moran VH, Stammers AL, Dillon S, Qualter P, Nissensohn M, Serra-Majem L, Lowe NM (2015) Zinc intake, status and indices of cognitive function in adults and children: a systematic review and meta-analysis. Eur J Clin Nutr 69:649–661. https://doi.org/10.1038/ejcn.2015.60
Pucheu S, Coudray C, Tresallet N, Favier A, de Leiris J (1995) Effect of dietary antioxidant trace element supply on cardiac tolerance to ischemia-reperfusion in the rat. J Mol Cell Cardiol 27:2303–2314. https://doi.org/10.1016/s0022-2828(95)91839-6
Wells EM, Bonfield TL, Dearborn DG, Jackson LW (2014) The relationship of blood lead with immunoglobulin E, eosinophils, and asthma among children: NHANES 2005–2006. Int J Hyg Environ Health 217:196–204. https://doi.org/10.1016/j.ijheh.2013.04.010
Wells G SB, O ’connell D, Peterson J, Welch V, Losos M, et al. (2009) The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta- analyses
Berkman ND, Lohr KN, Ansari M, McDonagh M, Balk E, Whitlock E, Reston J, Bass E, Butler M, Gartlehner G, Hartling L, Kane R, McPheeters M, Morgan L, Morton SC, Viswanathan M, Sista P, Chang S (2008) AHRQ Methods for Effective Health Care.
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Alsharnoubi J, Alkharbotly A, Waheed H, Elkhayat Z, Hussein DY (2020) Could we diagnose childhood asthma by LIBS technique? Lasers Med Sci 35:807–812. https://doi.org/10.1007/s10103-019-02866-6
Carneiro MFH, Rhoden CR, Amantea SL, Barbosa F (2011) Low concentrations of selenium and zinc in nails are associated with childhood asthma. Biol Trace Elem Res 144:244–252. https://doi.org/10.1007/s12011-011-9080-3
Cornwell CR, Egan KB, Zahran HS, Mirabelli MC, Hsu J, Chew GL (2020) Associations of blood lead levels with asthma and blood eosinophils in US children. Pediatr Allergy Immunol 31:695–699. https://doi.org/10.1111/pai.13241
Emeny RT, Korrick SA, Li Z, Nadeau K, Madan J, Jackson B, Baker E, Karagas MR (2019) Prenatal exposure to mercury in relation to infant infections and respiratory symptoms in the New Hampshire Birth Cohort Study. Environ Res 171:523–529. https://doi.org/10.1016/j.envres.2019.01.026
Grandjean P, Poulsen LK, Heilmann C, Steuerwald U, Weihe P (2010) Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ Health Perspect 118:1429–1433. https://doi.org/10.1289/ehp.1002289
Hon KL, Lui H, Wang SS, Lam HS, Leung TF (2012) Fish consumption, fish atopy and related heavy metals in childhood eczema. Iran J Allergy Asthma Immunol 11:230–235
Hon KL, Wang SS, Hung EC, Lam HS, Lui HH, Chow CM, Ching GK, Fok TF, Ng PC, Leung TF (2010) Serum levels of heavy metals in childhood eczema and skin diseases: friends or foes. Pediatr Allergy Immunol 21:831–836. https://doi.org/10.1111/j.1399-3038.2010.01022.x
Jedrychowski W, Perera F, Maugeri U, Miller RL, Rembiasz M, Flak E, Mroz E, Majewska R, Zembala M (2011) Intrauterine exposure to lead may enhance sensitization to common inhalant allergens in early childhood: a prospective prebirth cohort study. Environ Res 111:119–124. https://doi.org/10.1016/j.envres.2010.11.002
Joseph CL, Havstad S, Ownby DR, Peterson EL, Maliarik M, McCabe MJ Jr, Barone C, Johnson CC (2005) Blood lead level and risk of asthma. Environ Health Perspect 113:900–904. https://doi.org/10.1289/ehp.7453
Khafagy GM, Nada HR, Rashid LA, El-Samanoudy SI, Abd El-Sattar EM (2020) Role of trace elements in pityriasis Alba. J Trace Elem Med Biol 59:126422. https://doi.org/10.1016/j.jtemb.2019.126422
Kim J, Kim S, Woo SY, Chung JY, Hong YS, Oh SY, Choi SJ, Oh SY, Kim KW, Shin YH, Won HS, Lee KJ, Kim SH, Kwon JY, Lee SH, Hong SJ, Ahn K (2019) Prenatal exposure to lead and chromium is associated with IL-13 levels in umbilical cord blood and severity of atopic dermatitis: COCOA study. Immune Network 19. https://doi.org/10.4110/in.2019.19.e42
Heinrich J, Guo F, Trepka MJ (2017) Low-level mercury exposure and risk of asthma in school-age children. Epidemiology (Cambridge, Mass.) 28, 116–118. https://doi.org/10.1097/ede.0000000000000576
Kim KN, Bae S, Park HY, Kwon HJ, Hong YC (2015) Low-level mercury exposure and risk of asthma in school-age children. Epidemiology 26:733–739. https://doi.org/10.1097/ede.0000000000000351
Kocyigit A, Armutcu F, Gurel A, Ermis B (2004) Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace Elem Res 97:31–41. https://doi.org/10.1385/bter:97:1:31
Miyake Y, Tanaka K, Yasutake A, Sasaki S, Hirota Y (2011) Lack of association of mercury with risk of wheeze and eczema in Japanese children: the Osaka Maternal and Child Health Study. Environ Res 111:1180–1184. https://doi.org/10.1016/j.envres.2011.07.003
Mohammed AA, Mohamed FY, el El-Okda S, Ahmed AB (2015) Blood lead levels and childhood asthma. Indian Pediatr 52:303–306. https://doi.org/10.1007/s13312-015-0628-8
Myers SN, Rowell B, Binns HJ (2002) Lead poisoning and asthma: an examination of comorbidity. Arch Pediatr Adolesc Med 156:863–866. https://doi.org/10.1001/archpedi.156.9.863
Pesce G, Sese L, Calciano L, Travert B, Dessimond B, Maesano CN, Ferrante G, Huel G, Prud’homme J, Guinot M, Soomro MH, Baloch RM, Lhote R, Annesi-Maesano I (2021) Foetal exposure to heavy metals and risk of atopic diseases in early childhood. Pediatr Allergy Immunol 32:242–250. https://doi.org/10.1111/pai.13397
Rabito FA, Horter L, Langlois EC, Carlson JC, White LE, Schwartz K, Osman P, Rice JC (2013) Blood lead and pediatric asthma. Epidemiology 24:474–476. https://doi.org/10.1097/EDE.0b013e31828c7673
Razi CH, Akin O, Harmanci K, Akin B, Renda R (2011) Serum heavy metal and antioxidant element levels of children with recurrent wheezing. Allergol Immunopathol (Madr) 39:85–89. https://doi.org/10.1016/j.aller.2010.03.010
Shaheen SO, Newson RB, Henderson AJ, Emmett PM, Sherriff A, Cooke M, Team AS (2004) Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur Respir J 24:292–297. https://doi.org/10.1183/09031936.04.00117803
Shin J, Kim BM, Ha M, Park HS, Hong YC, Kim Y, Kwon JH, Ha EH (2019) The association between mercury exposure and atopic dermatitis in early childhood a mothers and children’s environmental health study. Epidemiology 30:S3–S8. https://doi.org/10.1097/ede.0000000000001002
Stelmach I, Grzelewski T, Bobrowska-Korzeniowska M, Kopka M, Majak P, Jerzynska J, Stelmach W, Polańska K, Sobala W, Gromadzińska J, Wa̧sowicz W, Hanke W, (2014) The role of zinc, copper, plasma glutathione peroxidase enzyme, and vitamins in the development of allergic diseases in early childhood: the Polish mother and child cohort study. Allergy Asthma Proc 35:227–232. https://doi.org/10.2500/aap.2014.35.3748
Toyran M, Kaymak M, Vezir E, Harmanci K, Kaya A, Ginis T, Kose G, Kocabas CN (2012) Trace element levels in children with atopic dermatitis. J Investig Allergol Clin Immunol 22:341–344
Uysalol M, Uysalol EP, Yilmaz Y, Parlakgul G, Ozden TA, Ertem HV, Omer B, Uzel N (2014) Serum level of vitamin D and trace elements in children with recurrent wheezing: a cross-sectional study. BMC Pediatr 14:270. https://doi.org/10.1186/1471-2431-14-270
Wang IJ, Karmaus WJJ, Yang CC (2017) Lead exposure, IgE, and the risk of asthma in children. J Expo Sci Environ Epidemiol 27:478–483. https://doi.org/10.1038/jes.2017.5
Weidinger S, Kramer U, Dunemann L, Mohrenschlager M, Ring J, Behrendt H (2004) Body burden of mercury is associated with acute atopic eczema and total IgE in children from Southem Germany. Journal of Allergy and Clinical Immunology 114:746–746. https://doi.org/10.1016/j.jaci.2004.04.011
Zeng X, Xu XJ, Zheng XB, Reponen T, Chen AM, Huo X (2016) Heavy metals in PM2.5 and in blood, and children’s respiratory symptoms and asthma from an e-waste recycling area. Environ Pollut 210:346–353. https://doi.org/10.1016/j.envpol.2016.01.025
Ozkan EA, Gocmen AY, Kucukbagriacik Y, Akyuz M (2019) Serum levels of trace elements, vitamin D and oxidant status in children with asthma. Journal of Basic and Clinical Health Sciences 3, 63–68. https://doi.org/10.30621/jbachs.2019.580
Smith PP, Nriagu JO (2011) Lead poisoning and asthma among low-income and African American children in Saginaw, Michigan. Environ Res 111:81–86. https://doi.org/10.1016/j.envres.2010.11.007
Bowler RP, Crapo JD (2002) Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol 110:349–356. https://doi.org/10.1067/mai.2002.126780
Fatani SH (2014) Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma 51:578–584. https://doi.org/10.3109/02770903.2014.892965
MacNee W (2001) Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 429:195–207. https://doi.org/10.1016/s0014-2999(01)01320-6
Guengerich FP (2018) Introduction to Metals in Biology 2018: copper homeostasis and utilization in redox enzymes. J Biol Chem 293:4603–4605. https://doi.org/10.1074/jbc.TM118.002255
Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186:1–85. https://doi.org/10.1016/0076-6879(90)86093-b
Samokyszyn VM, Miller DM, Reif DW, Aust SD (1989) Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem 264:21–26
Muñoz C, Rios E, Olivos J, Brunser O, Olivares M (2007) Iron, copper and immunocompetence. Br J Nutr 98(Suppl 1):S24–S28. https://doi.org/10.1017/s0007114507833046
Hopkins RG, Failla ML (1997) Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J Nutr 127:257–262. https://doi.org/10.1093/jn/127.2.257
Hopkins RG, Failla ML (1999) Transcriptional regulation of interleukin-2 gene expression is impaired by copper deficiency in Jurkat human T lymphocytes. J Nutr 129:596–601. https://doi.org/10.1093/jn/129.3.596
Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB (1998) Copper-dependent inflammation and nuclear factor-kappaB activation by particulate air pollution. Am J Respir Cell Mol Biol 19:366–378. https://doi.org/10.1165/ajrcmb.19.3.3042
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
EU (2007) Recommendation from the Scientific Committee on occupational exposure limits for elemental mercury and inorganic divalent mercury compounds. In: Work EUEaSAHaSa (Hrsg.)
Doth M, Fricke M, Nicoletti F, Garotta G, Van Velthuysen ML, Bruijn JA, Gleichmann E (1997) Genetic differences in immune reactivity to mercuric chloride (HgCl2): immunosuppression of H-2d mice is mediated by interferon-gamma (IFN-gamma). Clin Exp Immunol 109:149–156. https://doi.org/10.1046/j.1365-2249.1997.4041300.x
Dastych J, Walczak-Drzewiecka A, Wyczolkowska J, Metcalfe DD (1999) Murine mast cells exposed to mercuric chloride release granule-associated N-acetyl-beta-D-hexosaminidase and secrete IL-4 and TNF-alpha. J Allergy Clin Immunol 103:1108–1114. https://doi.org/10.1016/s0091-6749(99)70186-7
Toomey CB, Cauvi DM, Song WC, Pollard KM (2010) Decay-accelerating factor 1 (Daf1) deficiency exacerbates xenobiotic-induced autoimmunity. Immunology 131:99–106. https://doi.org/10.1111/j.1365-2567.2010.03279.x
Strenzke N, Grabbe J, Plath KE, Rohwer J, Wolff HH, Gibbs BF (2001) Mercuric chloride enhances immunoglobulin E-dependent mediator release from human basophils. Toxicol Appl Pharmacol 174:257–263. https://doi.org/10.1006/taap.2001.9223
de Vos G, Abotaga S, Liao Z, Jerschow E, Rosenstreich D (2007) Selective effect of mercury on Th2-type cytokine production in humans. Immunopharmacol Immunotoxicol 29:537–548. https://doi.org/10.1080/08923970701690993
Ancona A, Ramos M, Suarez R, Macotela E (1982) Mercury sensitivity in a dentist. Contact Dermatitis 8:218. https://doi.org/10.1111/j.1600-0536.1982.tb04198.x
Shin J, Kim BM, Ha M, Park HS, Hong YC, Kim Y, Hyun Kwon J, Ha EH (2019) The association between mercury exposure and atopic dermatitis in early childhood: a mothers and children’s environmental health study. Epidemiology 30(Suppl 1):S3-s8. https://doi.org/10.1097/ede.0000000000001002
Queiroz ML, Costa FF, Bincoletto C, Perlingeiro RC, Dantas DC, Cardoso MP, Almeida M (1994) Engulfment and killing capabilities of neutrophils and phagocytic splenic function in persons occupationally exposed to lead. Int J Immunopharmacol 16:239–244. https://doi.org/10.1016/0192-0561(94)90018-3
Krocova Z, Macela A, Kroca M, Hernychova L (2000) The immunomodulatory effect(s) of lead and cadmium on the cells of immune system in vitro. Toxicol In Vitro 14:33–40. https://doi.org/10.1016/s0887-2333(99)00089-2
Valentino M, Rapisarda V, Santarelli L, Bracci M, Scorcelletti M, Di Lorenzo L, Cassano F, Soleo L (2007) Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol 26:551–556. https://doi.org/10.1177/0960327107073817
Anetor JI, Adeniyi FA (1998) Decreased immune status in Nigerian workers occupationally exposed to lead. Afr J Med Med Sci 27:169–172
Yang SN, Hsieh CC, Kuo HF, Lee MS, Huang MY, Kuo CH, Hung CH (2014) The effects of environmental toxins on allergic inflammation. Allergy, Asthma Immunol Res 6:478–484. https://doi.org/10.4168/aair.2014.6.6.478
Min JY, Min KB, Kim R, Cho SI, Paek D (2008) Blood lead levels and increased bronchial responsiveness. Biol Trace Elem Res 123:41–46. https://doi.org/10.1007/s12011-008-8099-6
Funding
This work was supported by the National Natural Science Foundation of China grants (81872579, 81273123, 82173479), and the Graduate Student Scientific Practice Innovation Projects in Jiang Su province (KYCX19_0123).
Author information
Authors and Affiliations
Contributions
J. W., J. Y., and X. H.: literature search, screening, and data extraction. J. W., X. H., and J. Y.: data analysis and results visualization. J. W., J. Y., X. H., and R. L.: manuscript draft and modification. R. L.: fund acquirement. All authors reviewed the final version of the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Yin, J., Hong, X. et al. Exposure to Heavy Metals and Allergic Outcomes in Children: a Systematic Review and Meta-analysis. Biol Trace Elem Res 200, 4615–4631 (2022). https://doi.org/10.1007/s12011-021-03070-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03070-w