Abstract
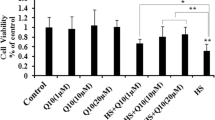
Heat stress, as a kind of oxidative stress, induces cell apoptosis. Apoptosis is a form of programmed cell death, and mitochondria play an important role in apoptosis. Manganese (Mn) has an antioxidant capacity by enhancing the activity of manganese superoxide dismutase (MnSOD). To investigate the potential effect of Mn on heat stress-induced apoptosis and mitochondrial function, we examined crucial related factors in the context of heat stress using primary chick embryonic myocardial cells pretreated with Mn for 24 h. The results showed that Mn restored the heat stress-induced decrease in cell viability and reduced the activities of caspase-3 (P < 0.05). The repression of the Δψm and intracellular ATP content caused by heat stress was reversed dramatically in the Mn pretreatment group (P < 0.05). Additionally, Mn inhibited heat stress-induced mitochondrial fission, as shown by decreased mitochondrial fission-related protein dynamin-related protein 1 (Drp1) expression and increased mitochondrial fusion-related protein optic atrophy 1 (Opa1) and mitofusin 1 (Mfn1) (P < 0.05) in primary chick embryonic myocardial cells. It was concluded that Mn attenuates the mitochondrial-mediated apoptosis pathway and sustains mitochondrial structure and function under heat stress in primary chick embryonic myocardial cells.






Similar content being viewed by others
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Change history
29 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12011-023-03652-w
References
Lara L, Rostagno M (2013) Impact of heat stress on poultry production. Animals 3(2):356–369. https://doi.org/10.3390/ani3020356
Chang CP, Hsu YC, Lin MT (2003) Magnolol protects against cerebral ischaemic injury of rat heatstroke. Clin Exp Pharmacol Physiol 30(5–6):387–392. https://doi.org/10.1046/j.1440-1681.2003.03847.x
Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V et al (2010) Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci 89(9):1905–1914. https://doi.org/10.3382/ps.2010-00812
Quinteiro-Filho WM, Gomes AV, Pinheiro ML et al (2012) Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella enteritidis. Avian Pathol 41(5):421–427. https://doi.org/10.1080/03079457.2012.709315
Kamel NN, Ahmed A, Mehaisen G et al (2017) Depression of leukocyte protein synthesis, immune function and growth performance induced by high environmental temperature in broiler chickens. Int J Biometeorol 61(9):1637–1645. https://doi.org/10.1007/s00484-017-1342-0
Slimen IB, Najar T, Ghram A et al (2014) Reactive oxygen species, heart stress and oxidative-induced mitoehondrial damage. A review. Int J Hyperthermia 30(7):513–523. https://doi.org/10.3109/02656736.2014.971446
Yang L, Tan GY, Fu YQ et al (2010) Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol 151(2):204–208. https://doi.org/10.1016/j.cbpc.2009.10.010
Zeng T, Li JJ, Wang DQ et al (2014) Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: evidence for differential thermal sensitivities. Cell Stress Chaperones 19(6):895–901. https://doi.org/10.1007/s12192-014-0514-7
Matsuki S et al (2003) Suppression of cytochrome c release and apoptosis in testes with heat stress by minocycline. Biochem Biophys Res Commun 312:843–849. https://doi.org/10.1016/j.bbrc.2003.10.191
Buckley IK (1972) A light and electron microscopic study of thermally injured cultured cells. Lab Invest 26(2):201–209
Sakaguchi Y, Stephens LC, Makino M, Kaneko T, Strebel FR et al (1995) Apoptosis in tumors and normal tissues induced by whole body hyperthermia in rats. Cancer Res 55:5459–5464
Bouchama A, Roberts G, Al MF, El-Sayed R, Lach B et al (1985) (2005) Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J Appl Physiol 98:697–705. https://doi.org/10.1152/japplphysiol.00461.2004
Di Wu Xu, Jiao SE et al (2015) Acetyl salicylic acid protected against heat stress damage in chicken myocardial cells and may associate with induced Hsp27 expression. Cell Stress Chaperones 20(4):687–696. https://doi.org/10.1007/s12192-015-0596-x
Islam A, Lv YJ, AbdeInasir A et al (2013) The role of Hsp90 alpha in heat-induced apoptosis and cell damage in; primary myocardial cell cultures of neonatal rats. Genet Mol Res 12(4):6080–6091. https://doi.org/10.4238/2013.december.2.6
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48(6):749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022
Trinei M, Migliaccio E, Bernardi P, Paolucci F, Pelicci P, Giorgio M (2013) p66Shc, mitochondria, and the generation of reactive oxygen species. Methods Enzymol 528:99–110. https://doi.org/10.1016/b978-0-12-405881-1.00006-9
Gu ZT, Wang H, Li L, Liu YS, Deng XB, Huo SF, Yuan FF, Liu ZF, Tong HS, Su L (2014) Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci Rep 4:4469. https://doi.org/10.1038/srep04469
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8(11):870–879. https://doi.org/10.1038/nrm2275
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99. https://doi.org/10.1146/annurev.cellbio.22.010305.104638
Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22(12):1577–1590. https://doi.org/10.1101/gad.1658508
Jian F, Chen D, Chen L, Yan C, Lu B, Zhu Y et al (2018) Sam50 regulates PINK1-Parkin-mediated mitophagy by controlling PINK1 stability and mitochondrial morphology. Cell Rep 23(10):2989–3005. https://doi.org/10.1016/j.celrep.2018.05.015
Hitchler M, Wikainapakul K, Yu L et al (2006) Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics 1(4):163–171. https://doi.org/10.4161/epi.1.4.3401
Gu ZT, Li L, Wu F, Zhao P, Yang H et al (2015) Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci Rep 5:11497. https://doi.org/10.1038/srep11497
Li L, Tan H, Gu Z et al (2014) Heat stress induces apoptosis through a Ca2+-mediated mitochondrial apoptotic pathway in human umbilical vein endothelial cells. PLoS ONE 9(12):e111083. https://doi.org/10.1371/journal.pone.0111083
Qin SZ, Liao XD, Lu L, Zhang LY, Xi L, Guo YL, Luo XG (2017) Manganese enhances the expression of the manganese superoxide dismutase in cultured primary chick embryonic myocardial cells. J Integr Agric 16(9):2038–2046. https://doi.org/10.1016/S2095-3119(16)61527-7
Qin SZ, Wang R, Tang DF, Qin SJ, Guo YL, Shi ZG (2021) Manganese mitigates heat stress-induced apoptosis by alleviating endoplasmic reticulum stress and activating the NRF2/SOD2 pathway in primary chick embryonic myocardial cells. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02810-2
Vanmuylder N, Evrard L, Daelemans P, Van RJ, Dourov N (2000) Immunohisto chemical expression of heat shock proteins HSP27, HSP70, HSP90 and HSP110 in salivary gland tumors: a study of 50 cases. Ann Pathol 20(3):190–195
Xiong YJ, Yin QR, Jin EH, Chen HT, He SJ (2020) Selenium attenuates chronic heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in mouse granulosa cells. Molecules 25:557
Roberts GT et al (2008) Microvascular injury, thrombosis, inflammation, and apoptosis in the pathogenesis of heatstroke a study in baboon model. Arterioscler Thromb Vasc Biol 28:1130–1136. https://doi.org/10.1161/atvbaha.107.158709
Oberley LW, Buettner GR (1979) Role of superoxide dismutase in cancer: a review. Cancer Res 39(4):1141–1149
Hsu YL et al (2011) Heat shock induces apoptosis through reactive oxygen speciesinvolving mitochondrial and death receptor pathways in corneal cells. Exp Eye Res 93(4):405–412. https://doi.org/10.1016/j.exer.2011.06.005
Milleron RS, Bratton SB (2006) Heat shock induces apoptosis independently of any known initiator caspase-activating complex. J Biol Chem 281(25):16991–17000. https://doi.org/10.1074/jbc.m512754200
Fan X, Hussien R, Brooks GA (2010) H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49(11):1646–1654. https://doi.org/10.1016/j.freeradbiomed.2010.08.024
Wu S, Zhou F, Zhang Z, Xing D (2011) Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fissionfusion proteins. FEBS J 278(6):941–954. https://doi.org/10.1111/j.1742-4658.2011.08010.x
Nagdas S, Kashatus DF (2017) The interplay between oncogenic signaling networks and mitochondrial dynamics. Antioxidants 6(2):33. https://doi.org/10.3390/antiox6020033
Wang W, Wang R, Zhang Q, Mor G, Zhang H (2018) Benzo(a)pyren-7,8- dihydrodiol -9,10-epoxide induces human trophoblast Swan 71 cell dysfunctions due to cell apoptosis through disorder of mitochondrial fission/fusion. Environ Pollut 233:820–832. https://doi.org/10.1016/j.envpol.2017.11.022
Jafri MS, Dudycha SJ, O’Rourke B (2001) Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng 3:57–81. https://doi.org/10.1146/annurev.bioeng.3.1.57
Burman JL, Itsara LS, Kayser EB, Suthammarak W, Wang AM et al (2014) A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech 7(10):1165–1174. https://doi.org/10.1242/dmm.015321
Callista Y, Wen Y, Siegfried H (2014) The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157(4):897–909. https://doi.org/10.1016/j.cell.2014.02.055
Wang HL, Xing GD, Qian Y, Sun XF, Zhong JF, Chen KL (2021) Dihydromyricetin attenuates heat stress-induced apoptosis in dairy cow mammary epithelial cells through suppressing mitochondrial dysfunction. Ecotoxicol Environ Saf 214:112078. https://doi.org/10.1016/j.ecoenv.2021.112078
Zhao QL, Fujiwara Y, Kondo T (2006) Mechanism of cell death induction by nitroxide and hyperthermia. Free Radic Biol Med 40(7):1131–1143. https://doi.org/10.1016/j.freeradbiomed.2005.10.064
Qi D, Yong LH (2015) AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab 26(8):422–429. https://doi.org/10.1016/j.tem.2015.05.010
Velagapudi R, El-bakoush A, Lepiarz I et al (2017) AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol Cell Biochem 435(1–2):149–162. https://doi.org/10.1007/s11010-017-3064-3
Bröer A, Juelich T, Vanslambrouck JM, Tietze N, Solomon PS, Holst J et al (2011) Impaired nutrient signaling and body weight control in a Na+ neutral amino acid cotransporter (Slc6a19)-deficient mouse. J Biol Chem 286(30):26638–26651. https://doi.org/10.1074/jbc.m111.241323
Choi J, Chandrasekaran K, Inoue T et al (2014) PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol Dis 64:118–130. https://doi.org/10.1016/j.nbd.2014.01.001
Huang TY, Zheng DH, Houmard JA et al (2017) Overexpression of PGC-1α increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am J Physiol Endocrinol Metab 312(4):E253–E263. https://doi.org/10.1152/ajpendo.00331.2016
Lopaschuk GD, Ussher JR, Folmes CDL et al (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1):207–258. https://doi.org/10.1152/physrev.00015.2009
Funding
This research was funded by National Natural Science Foundation of China (No. 03119023).
Author information
Authors and Affiliations
Contributions
Rui Wang: design, investigation and writing-original draft; Zhaoguo Shi: project administration; Jinlu Li and Defu Tang: contributed materials; Yanli Guo: conceptualization and writing (review and editing); Shizhen Qin: conceptualization and funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethics approval
All procedures were approved by the Animal Welfare Committee of the Institute of Animal Science, Gansu Agricultural University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Shi, Z., Li, J. et al. Protective Effect of Manganese on Apoptosis and Mitochondrial Function of Heat-Stressed Primary Chick Embryonic Myocardial Cells. Biol Trace Elem Res 200, 4419–4429 (2022). https://doi.org/10.1007/s12011-021-03016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03016-2