Abstract
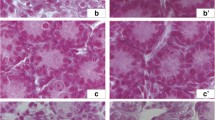
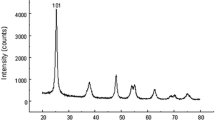
Cerium oxide (CeO2) has potential applications in medicine and various consumer products. This study investigated the effect of CeO2 on the expression of genes associated with apoptosis and testicular development in mouse embryos. The experimental groups of pregnant mice were injected intraperitoneally with CeO2 at a concentration of 10 mg/kg on days 7 and 14 of pregnancy. Six days after birth, the testicles of neonatal male mice were collected for mRNA expression determination using real-time PCR, protein expression analysis by immunohistochemistry, and apoptotic cell population determination using the TUNEL assay. The results showed that the mRNA expression of the Bax, Caspase-3, and Gsk3-β genes, unlike the Bcl2 gene, decreased significantly in the experimental group compared to the control group. The expression ratio of Bax/Bcl2 in the experimental group was lower than in the control group. A similar trend was observed in the population of apoptotic cells. In the experimental group, the expression levels of, Gata4, Sox8, and Rad54 at both the mRNA and protein levels increased significantly compared to the control group. Based on the results of this study, CeO2 at a concentration of 10 mg/kg, in addition to producing anti-apoptotic effects on the testicular cells of neonatal mice, can increase the expression of genes involved in testicular development and performance. The current experimental study proved the protective effects of 10 mg/kg CeO2 in developmental and apoptosis genes of testicular tissue in 6-day-old NMRI mice fetuses; however, more experiments are required to evaluate the possible side effects and interactions.






Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Estelrich J, Sánchez-Martín MJ, Busquets MA (2015) Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomed 10:1727
Polak P, Shefi O (2015) Nanometric agents in the service of neuroscience: manipulation of neuronal growth and activity using nanoparticles. Nanomed Nanotechnol Biol Med 11(6):1467–1479
Kawagoe M, Ishikawa K, Wang S-C, Yoshikawa K, Arany S, Zhou X-P et al (2008) Acute effects on the lung and the liver of oral administration of cerium chloride on adult, neonatal and fetal mice. J Trace Elem Med Biol 22(1):59–65
Dunnick KM, Morris AM, Badding MA, Barger M, Stefaniak AB, Sabolsky EM et al (2016) Evaluation of the effect of valence state on cerium oxide nanoparticle toxicity following intratracheal instillation in rats. Nanotoxicology 10(7):992–1000
Nemati A, Farhadi A, Jalili C, Gholami M (2019) The effect of cerium oxide during pregnancy on the development of the testicular tissue of newborn NMRI mice. Biol Trace Elem Res 195:196–204
Nemati A, Assadollahi V, Peluso I, Abbaszadeh A, Beigi-Boroujeni M, Khanipur Z et al (2020) A stereological study of the toxic effects of cerium oxide during pregnancy on kidney tissues in neonatal NMRI mice. Oxid Med Cell Longev 2020:1–11
Nemati A, Beyranvand F, Assadollahi V, Salahshoor MR, Alasvand M, Gholami MR (2021) The effect of different concentrations of cerium oxide during pregnancy on ovarian follicle development in neonatal mice. Birth Defects Res 113(4):349–358
Falchi L, Bogliolo L, Galleri G, Ariu F, Zedda MT, Pinna A et al (2016) Cerium dioxide nanoparticles did not alter the functional and morphologic characteristics of ram sperm during short-term exposure. Theriogenology 85(7):1274-1281. e3
Shi L, Yue W, Zhang C, Ren Y, Zhu X, Wang Q et al (2010) Effects of maternal and dietary selenium (Se-enriched yeast) on oxidative status in testis and apoptosis of germ cells during spermatogenesis of their offspring in goats. Anim Reprod Sci 119(3–4):212–218
Sisakhtnezhad S, Bahrami AR, Moghadam Matin M, Behnam Rassouli F, Momeni-Moghadam M, Boozarpour S (2016) Spermatogonial stem cells: biology, isolation, culture, characterization, and practical perspectives. J Kerman Univ Med Sci 23(6):797–828
Schrade A, Kyrönlahti A, Akinrinade O, Pihlajoki M, Häkkinen M, Fischer S et al (2015) GATA4 is a key regulator of steroidogenesis and glycolysis in mouse Leydig cells. Endocrinology 156(5):1860–1872
Takada S, Koopman P (2003) Origin and possible roles of the Sox8 transcription factor gene during sexual development. Cytogenet Genome Res 101(3–4):212–218
Jin J-Z, Ding J (2006) Analysis of cell migration, transdifferentiation and apoptosis during mouse secondary palate fusion. Development 133(17):3341–3347
Thornberry NA (1997) The caspase family of cysteine proteases. Br Med Bull 53(3):478–490
Messiaen S, Le Bras A, Duquenne C, Barroca V, Moison D, Déchamps N et al (2013) Rad 54 is required for the normal development of male and female germ cells and contributes to the maintainance of their genome integrity after genotoxic stress. Cell Death Dis 4(8):e774-e
Kim L, Kimmel AR (2006) GSK3 at the edge: regulation of developmental specification and cell polarization. Curr Drug Targets 7(11):1411–1419
Frame S, Cohen P (2001) The renaissance of GSK3. Nat Rev Mol Cell Biol 2(10):769–776
Frame S, Cohen P (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359(1):1–16
Hetman M, Cavanaugh JE, Kimelman D, Xia Z (2000) Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20(7):2567–2574
Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA et al (2004) Glycogen synthase kinase-3β phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24(44):9993–10002
Nishimura H, Nakamura O, Yamagami Y, Mori M, Horie R, Fukuoka N et al (2016) GSK-3 inhibitor inhibits cell proliferation and induces apoptosis in human osteosarcoma cells. Oncol Rep 35(4):2348–2354
Rashid M, Mazur T, Ji W, Liu S, Taylor W (2018) Analysis of the role of GSK3 in the mitotic checkpoint. Sci Rep 8(1):1–16
Moyano DF, Rotello VM (2011) Nano meets biology: structure and function at the nanoparticle interface. Langmuir 27(17):10376–10385
Shinohara T, Orwig KE, Avarbock MR, Brinster RL (2001) Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci 98(11):6186–6191
Clermont Y, Perey B (1957) Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 100(2):241–267
Nagano M, Brinster R (1998) Spermatogonial transplantation and reconstitution of donor cell spermatogenesis in recipient mice. APMIS 106(1–6):47–57
McCarrey JR (2013) Toward a more precise and informative nomenclature describing fetal and neonatal male germ cells in rodents. Biol Reprod 89(2):47, 1–9
McLean DJ, Friel PJ, Johnston DS, Griswold MD (2003) Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol Reprod 69(6):2085–2091
Park K, Park J, Lee H, Choi J, Yu W-J, Lee J (2018) Toxicity and tissue distribution of cerium oxide nanoparticles in rats by two different routes: single intravenous injection and single oral administration. Arch Pharmacal Res 41(11):1108–1116
Khorrami MB, Sadeghnia HR, Pasdar A, Ghayour-Mobarhan M, Riahi-Zanjani B, Hashemzadeh A et al (2019) Antioxidant and toxicity studies of biosynthesized cerium oxide nanoparticles in rats. Int J Nanomed 14:2915
Inupakutika MA, Sengupta S, Devireddy AR, Azad RK, Mittler R (2016) The evolution of reactive oxygen species metabolism. J Exp Bot erw382 67(21):5933–5943
Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC (2000) The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 21(6):895–902
Saikia H, Hazarika KK, Chutia B, Choudhury B, Bharali P (2017) A simple chemical route toward high surface area CeO2 nanoparticles displaying remarkable radical scavenging activity. ChemistrySelect 2(11):3369–3375
Bedner E, Li X, Kunicki J, Darzynkiewicz Z (2000) Translocation of Bax to mitochondria during apoptosis measured by laser scanning cytometry. Cytometry 41(2):83–88
Rostami S, Abroun S, Alimoghaddam K, Nia MN, Chahardouli B, Ghavamzadeh A et al (2013) XML Arsenic trioxideinduced apoptosis in HL-60 cell line by decreasing the ratio of Bcl-2 to Bax mRNA expression. Sci J Iran Blood Transfus Organ 9(4):363–371
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116(7):1175–1186
Beurel E, Jope RS (2006) The paradoxical pro-and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79(4):173–189
Khanehzad M, Abolhasani F, Koruji SM, Ragerdi Kashani I, Aliakbari F (2016) The roles of Sertoli cells in fate determinations of spermatogonial stem cells. Tehran Univ Med J TUMS Publ 73(12):878–887
Chen S-R, Tang J-X, Cheng J-M, Li J, Jin C, Li X-Y et al (2015) Loss of Gata4 in Sertoli cells impairs the spermatogonial stem cell niche and causes germ cell exhaustion by attenuating chemokine signaling. Oncotarget 6(35):37012
Hasanzadeh S, Najafi G, Pirdehghan HR, Bonyadi F, Zavvary M, Ebrahimi F et al (2016) XML The effects of ceftriaxone on histology, histomorphometry, and histochemistry of testis and sperm characteristics in mice. Qom Univ Med Sci J 10(6):1–12
Lejeune H, Skalli M, Chatelain PG, Avallet O, Saez JM (1992) The paracrine role of Sertoli cells on Leydig cell function. Cell Biol Toxicol 8(3):73–83
O’Bryan MK, Takada S, Kennedy CL, Scott G, Harada S-I, Ray MK et al (2008) Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol 316(2):359–370
Moridi H, Hosseini SA, Shateri H, Kheiripour N, Kaki A, Hatami M et al (2018) Protective effect of cerium oxide nanoparticle on sperm quality and oxidative damage in malathion-induced testicular toxicity in rats: an experimental study. Int J Reprod Biomed 16(4):261
Acknowledgements
We express our appreciation to all members of the Cellular and Molecular Research Center for their helpful consultation and deliberation during this work.
Funding
Kurdistan University of Medical Sciences (No. IR.MUK.REC.1400.094).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the experimental design and performed experiments and also contributed to data analysis, interpretation of the results, and drafting of the manuscript. The main responsible for the drafting of the manuscript was Vahideh Assadollahi and Masoud Alasvand.
Corresponding authors
Ethics declarations
Ethical Approval
All stages of this experiment are in accordance with the ethical standards of the Ethics Committee of Kurdistan University of Medical Sciences, Sanandaj, Iran (Approval ID IR.MUK.REC.1400.094).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amiri, G., Gholami, M., Assadollahi, V. et al. Effect of Cerium Oxide Nanoparticles on the Expression of Developmental and Apoptosis Genes of Testicular Tissue in 6-Day-Old NMRI Mice Fetuses. Biol Trace Elem Res 200, 3265–3274 (2022). https://doi.org/10.1007/s12011-021-02939-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02939-0