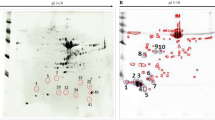
Abstract
In recent decades, the scientific community has widely debated the contamination of fish in the Amazon region by mercury species. As the diet of riverside populations in the Amazon region is based mainly on fish, these populations are exposed to mercurial species that can cause serious and irreversible damage to their health. The risks of consuming fish exposed to mercurial species in the Amazon region have motivated toxicological investigations. However, the effect of mercurial species on protein and enzyme levels is still controversial. In this work, analytical and bioanalytical techniques Two-dimensional polyacrylamide gel electrophoresis [2D-PAGE] Graphite Furnace Atomic Absorption Spectrometry [GFAAS], and Mass Spectrometry in Sequence with Electrospray Ionization [ESI–MS/MS] were used to identify proteins associated with mercury (metal-binding protein) in muscle and liver tissues of the fish species Pinirampus pirinampu from the Madeira River, in the Brazilian Amazon. Enzymatic and lipid peroxidation analyses were also used to assess changes related to oxidative stress. Determinations of total mercury by GFAAS indicated higher concentrations in liver tissue (555 ± 19.0 µg kg−1) when compared to muscle tissue (60 ± 2.0 µg kg−1). The fractionation process of tissue proteomes by 2D-PAGE and subsequent mapping of mercury by GFAAS in the protein spots of the gels identified the presence of mercury in three spots of the liver tissue (concentrations in the range of 0.800 to 1.90 mg kg−1). The characterization of protein spots associated with mercury by ESI–MS/MS identified the enzymes triosephosphate isomerase A, adenylate kinase 2 mitochondrial, and glyceraldehyde-3-phosphate dehydrogenase as possible candidates for mercury exposure biomarkers. The muscle tissue did not show protein spots associated with mercury. Enzymatic activity decreased proportionally to the increase in mercury concentrations in the tissues.
Graphical abstract




Similar content being viewed by others
Data and materials availability
All raw data will be made available on request.
Code Availability
Not applicable.
References
Maniero MÁ, Wuilloud RG, Callegari EA et al (2020) Metalloproteomics analysis in human mammary cell lines treated with inorganic mercury. J Trace Elem Med Biol 58:126441. https://doi.org/10.1016/j.jtemb.2019.126441
Kahhat R, Parodi E, Larrea-Gallegos G et al (2019) Environmental impacts of the life cycle of alluvial gold mining in the Peruvian Amazon rainforest. Sci Total Environ 662:940–951. https://doi.org/10.1016/j.scitotenv.2019.01.246
Alcala-Orozco M, Caballero-Gallardo K, Olivero-Verbel J (2019) Mercury exposure assessment in indigenous communities from Tarapaca village, Cotuhe and Putumayo Rivers, Colombian Amazon. Environ Sci Pollut Res 26:36458–36467. https://doi.org/10.1007/s11356-019-06620-x
Vieira JCS, de Oliveira G, Braga CP et al (2020) Parvalbumin and ubiquitin as potential biomarkers of mercury contamination of Amazonian Brazilian fish. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02026-w
Zheng N, Wang S, Dong W et al (2019) The toxicological effects of mercury exposure in marine fish. Bull Environ Contam Toxicol 102:714–720. https://doi.org/10.1007/s00128-019-02593-2
Le DQ, Tanaka K, Dung LV et al (2017) Biomagnification of total mercury in the mangrove lagoon foodweb in east coast of Peninsula, Malaysia. Regional Studies in Marine Science 16:49–55. https://doi.org/10.1016/j.rsma.2017.08.006
Raihan SM, Moniruzzaman M, Park Y et al (2020) Evaluation of dietary organic and inorganic mercury threshold levels on induced mercury toxicity in a marine fish model. Animals 10:405. https://doi.org/10.3390/ani10030405
Passos CJS, Mergler D (2008) Human mercury exposure and adverse health effects in the Amazon: a review. Cad Saude Publica 24:s503–s520. https://doi.org/10.1590/S0102-311X2008001600004
Yang L, Zhang Y, Wang F et al (2020) Toxicity of mercury: molecular evidence. Chemosphere 245:125586. https://doi.org/10.1016/j.chemosphere.2019.125586
Crump KL, Trudeau VL (2009) Mercury-induced reproductive impairment in fish. Environ Toxicol Chem 28:895. https://doi.org/10.1897/08-151.1
Tan SW, Meiller JC, Mahaffey KR (2009) The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol 39:228–269. https://doi.org/10.1080/10408440802233259
Sandheinrich MB, Miller KM (2006) Effects of dietary methylmercury on reproductive behavior of fathead minnows (Pimephales promelas). Environ Toxicol Chem 25:3053. https://doi.org/10.1897/05-641R.1
Batchelar KL, Kidd KA, Drevnick PE et al (2013) Evidence of impaired health in yellow perch (Perca flavescens ) from a biological mercury hotspot in northeastern north America. Environ Toxicol Chem 32:627–637. https://doi.org/10.1002/etc.2099
Ynalvez R, Gutierrez J, Gonzalez-Cantu H (2016) Mini-review: toxicity of mercury as a consequence of enzyme alteration. Biometals 29:781–788. https://doi.org/10.1007/s10534-016-9967-8
Vieira JCS, Cavecci B, Queiroz JV et al (2015) Determination of the mercury fraction linked to protein of muscle and liver tissue of Tucunaré (Cichla spp.) from the Amazon Region of Brazil. Arch Environ Contam Toxicol 69:422–430. https://doi.org/10.1007/s00244-015-0160-9
Braga CP, Bittarello AC, Padilha CCF et al (2015) Mercury fractionation in dourada (Brachyplatystoma rousseauxii) of the Madeira River in Brazil using metalloproteomic strategies. Talanta 132:239–244. https://doi.org/10.1016/j.talanta.2014.09.021
da Cunha Bataglioli I, Souza Vieira JC, Vitor de Queiroz J et al (2019) Physiological and functional aspects of metal-binding protein associated with mercury in the liver tissue of pirarucu (Arapaima gigas) from the Brazilian Amazon. Chemosphere 236:124320. https://doi.org/10.1016/j.chemosphere.2019.07.051
de Queiroz JV, Vieira JCS, de Oliveira G et al (2019) Identification of biomarkers of mercury contamination in Brachyplatystoma filamentosum of the Madeira River, Brazil, using metalloproteomic strategies. Biol Trace Elem Res 187:291–300. https://doi.org/10.1007/s12011-018-1363-5
Bittarello AC, Vieira JCS, Braga CP et al (2019) Characterization of molecular biomarkers of mercury exposure to muscle tissue of Plagioscion squamosissimus and Colossoma macropomum from the Amazon region. Food Chem 276:247–254. https://doi.org/10.1016/j.foodchem.2018.10.002
Cavecci-Mendonça B, de Souza C, Vieira J, Monteiro de Lima P et al (2020) Study of proteins with mercury in fish from the Amazon region. Food Chem 309:125460. https://doi.org/10.1016/j.foodchem.2019.125460
Queiroz LJ, Vilara-Torrente G, Ohara WM, Pires THS, Zuanon J, Doria CRC (2013) Peixes do Rio Madeira.
Bittarello AC, Vieira JCS, Braga CP et al (2020) Metalloproteomic approach of mercury-binding proteins in liver and kidney tissues of Plagioscion squamosissimus (corvina) and Colossoma macropomum (tambaqui) from Amazon region: possible identification of mercury contamination biomarkers. Sci Total Environ 711:134547. https://doi.org/10.1016/j.scitotenv.2019.134547
Moraes PM, Santos FA, Cavecci B et al (2013) GFAAS determination of mercury in muscle samples of fish from Amazon, Brazil. Food Chem 141:2614–2617. https://doi.org/10.1016/j.foodchem.2013.05.008
Silva F, Padilha C, Pezzato L et al (2006) Determination of chromium by GFAAS in slurries of fish feces to estimate the apparent digestibility of nutrients in feed used in pisciculture. Talanta 69:1025–1030. https://doi.org/10.1016/j.talanta.2005.12.008
Silva FA, Neves RCF, Quintero-Pinto LG et al (2007) Determination of selenium by GFAAS in slurries of fish feces to estimate the bioavailability of this micronutrient in feed used in pisciculture. Chemosphere 68:1542–1547. https://doi.org/10.1016/j.chemosphere.2007.03.003
Jiang Z-Y, Woollard ACS, Wolff SP (1991) Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 26:853–856. https://doi.org/10.1007/BF02536169
Crouch RK, Gandy SE, Kimsey G et al (1981) The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes 30:235–241. https://doi.org/10.2337/diab.30.3.235
Beutler E (1975) Red cell metabolism: a manual of biochemical a methods New York
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
de Queiroz JV, Vieira JCS, da Cunha BI et al (2018) Total mercury determination in muscle and liver tissue samples from Brazilian Amazon fish using slurry sampling. Biol Trace Elem Res 184:517–522. https://doi.org/10.1007/s12011-017-1212-y
Doumas BT, Bayse DD, Borner K et al (1981) A candidate reference method for determination of total protein in serum II. Test for transferability Clinical Chemistry 271027:1651–1654
Vieira JCS, Braga CP, de Oliveira G et al (2018) Correction to: Mercury exposure: protein biomarkers of mercury exposure in Jaraqui Fish from the Amazon Region. Biol Trace Elem Res 183:172. https://doi.org/10.1007/s12011-017-1195-8
Lima PM, Neves RDCF, dos Santos F, a, et al (2010) Analytical approach to the metallomic of Nile tilapia (Oreochromis niloticus) liver tissue by SRXRF and FAAS after 2D-PAGE separation: Preliminary results. Talanta 82:1052–1056. https://doi.org/10.1016/j.talanta.2010.06.023
Shevchenko A, Tomas H, Havliš J et al (2007) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. https://doi.org/10.1038/nprot.2006.468
Uniprot (2019) Uniprot.org.br. 2019 1.
Home | BioBam | Bioinformatics Made Easy. https://www.biobam.com/. Accessed 20 Apr 2020
de Queiroz JV, Cavecci-Mendonça B, Vieira JCS et al (2021) Metalloproteomic strategies for identifying proteins as biomarkers of mercury exposure in Serrasalmus rhombeus from the Amazon Region. Biol Trace Elem Res 199:712–720. https://doi.org/10.1007/s12011-020-02178-9
Marinho JS, Lima MO, de Santos EC, O, et al (2014) Mercury speciation in hair of children in three communities of the Amazon, Brazil. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/945963
Rodríguez Martín-Doimeadios RC, Berzas Nevado JJ, Guzmán Bernardo FJ et al (2014) Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ Sci Pollut Res 21:7466–7479. https://doi.org/10.1007/s11356-014-2680-7
Souza-Araujo J, Giarrizzo T, Lima MO, Souza MBG (2016) Mercury and methyl mercury in fishes from Bacajá River (Brazilian Amazon): evidence for bioaccumulation and biomagnification. J Fish Biol 89:249–263. https://doi.org/10.1111/jfb.13027
Cerbino MR, Vieira JCS, Braga CP et al (2017) Metalloproteomics approach to analyze mercury in breast milk and hair samples of lactating women in communities of the Amazon Basin Brazil Biological Trace Element Research 1–11 https://doi.org/10.1007/s12011-017-1057-4
dos Santos FA, Cavecci B, Vieira JCS et al (2015) A metalloproteomics study on the association of mercury with breast milk in samples from lactating women in the Amazon Region of Brazil. Arch Environ Contam Toxicol 69:223–229. https://doi.org/10.1007/s00244-015-0161-8
Kumar A, Khushboo PR, Sharma B (2020) Modulation of superoxide dismutase activity by mercury, lead, and arsenic. Biol Trace Elem Res 196:654–661. https://doi.org/10.1007/s12011-019-01957-3
Ibrahim ATA, Banaee M, Sureda A (2019) Selenium protection against mercury toxicity on the male reproductive system of Clarias gariepinus. Comp Biochem Physiol C: Toxicol Pharmacol 225:108583. https://doi.org/10.1016/j.cbpc.2019.108583
Grotto D, Vicentini J, Friedmann Angeli JP et al (2011) Evaluation of protective effects of fish oil against oxidative damage in rats exposed to methylmercury. Ecotoxicol Environ Saf 74:487–493. https://doi.org/10.1016/j.ecoenv.2010.10.012
Brandão F, Cappello T, Raimundo J et al (2015) Unravelling the mechanisms of mercury hepatotoxicity in wild fish (Liza aurata) through a triad approach: bioaccumulation, metabolomic profiles and oxidative stress. Metallomics 7:1352–1363. https://doi.org/10.1039/C5MT00090D
www.uniprot (2021) tpi1a - Triosephosphate isomerase A - Danio rerio (Zebrafish) - tpi1a gene & protein. 1.
Uniprot.org/uniprot (2021) ak2 - Adenylate kinase 2, mitochondrial - Danio rerio (Zebrafish) - ak2 gene & protein. 1.
Burkart A, Shi X, Chouinard M, Corvera S (2011) Adenylate kinase 2 links mitochondrial energy metabolism to the induction of the unfolded protein response. J Biol Chem 286:4081–4089. https://doi.org/10.1074/jbc.M110.134106
Klepinin A, Zhang S, Klepinina L, et al (2020) Adenylate kinase and metabolic signaling in cancer cells. frontiers in oncology. doi: https://doi.org/10.3389/fonc.2020.00660
Rissone A, Weinacht KG, la Marca G et al (2015) Reticular dysgenesis–associated AK2 protects hematopoietic stem and progenitor cell development from oxidative stress. J Exp Med 212:1185–1202. https://doi.org/10.1084/jem.20141286
Uniprot.org (2021) gapdh - Glyceraldehyde-3-phosphate dehydrogenase - Danio rerio (Zebrafish) - gapdh gene & protein. https://www.uniprot.org/uniprot/Q5XJ10. Accessed 18 Mar 2021
Wassarman PM, Watson HC, Major JP (1969) Reaction of the sulphydryl groups of lobster-muscle glyceraldehyde-3-phosphate dehydrogenase with organic mercurials. BBA - Enzymology 191:1–9. https://doi.org/10.1016/0005-2744(69)90309-X
BioBam Bioinformatics (2019) OmicsBox – Bioinformatics Made Easy. March 3, 2019.
www.blast2go.com (2017) Blast2GO - Functional Annotation and Genomics. 2017 1.
Dufault R, Schnoll R, Lukiw WJ et al (2009) Mercury exposure, nutritional deficiencies and metabolic disruptions may affect learning in children. Behav Brain Funct 5:44. https://doi.org/10.1186/1744-9081-5-44
Funding
The authors thank the Brazilian research-funding agency: National Electric Energy Agency-ANEEL/Sustainable Energy of Brazil-ESBR – P&D: 6631–0001/2012/Contract Jirau 004/2013, São Paulo Research Foundation-FAPESP, Processes: 2016/19404–2 and 2014/02668–1), National Council for Scientific and Technological Development–CNPq, Processes: 404485/2016–2, 303719/2014–1 and 30478/2018–9) and CAPES-Print AUXPE-Process: 88881.3107432018–01 for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: José Cavalcante Souza Vieira, Camila Pereira Braga and Pedro de Magalhaes Padilha; Methodology: José Cavalcante Souza Vieira, Grasieli de Oliveira, Camila Pereira Braga, Nubya Gonçalves Cavallini, Luiz Fabrício Zara, Marília Afonso Rabelo Buzalaf, Pedro de Magalhães Padilha; Formal analysis and investigation: José Cavalcante Souza Vieira, Grasieli de Oliveira, Pedro Magalhãe Padilha; Writing—original draft preparation: José Cavalcante Souza Vieira, Grasieli de Oliveira, Nubya Gonçalves Cavallini; Writing—review and editing: Pedro de Magalhães padilha, Camila Pereira Braga, Jiri Adamec; Funding acquisition: Pedro de Magalhães Padilha, José Cavalcante Souza Vieira; Resources: Pedro de Magalhães Padilha, José Cavalcante Souza Vieira; Supervision: Pedro de Magalhães Padilha.
Corresponding authors
Ethics declarations
Ethics approval
The protocols used in this study were approved by the Experimental Animal Ethics Committee at São Paulo State University (UNESP), Botucatu, São Paulo, under protocol CEUA n° 186/2017.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vieira, J.C.S., de Oliveira, G., Cavallini, N.G. et al. Investigation of Protein Biomarkers and Oxidative Stress in Pinirampus pirinampu Exposed to Mercury Species from the Madeira River, Amazon-Brazil. Biol Trace Elem Res 200, 1872–1882 (2022). https://doi.org/10.1007/s12011-021-02805-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02805-z