Abstract
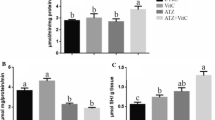
The present experiment was designed to evaluate the effect of graded level of zinc on Vitellogenin gene (Vtg) expression and antioxidant enzymes in threatened catfish, Clarias magur (C. magur). One hundred and eighty female C. magur with an average weight of 145 ± 5 g were allocated in twelve cemented tanks with dimension 4.5 × 2 × 1 m for a period of 60 days. Fish were distributed in four groups with three replicates following the completely randomised design. The first group treated as control (C) fed with basal diet contained normal zinc level, and remaining groups were fed with basal diets having 50, 200 and 300 mg/kg zinc acetate and treated as T1, T2 and T3 respectively. To evaluate the effect of dietary zinc supplementation on Vtg gene expression, three sampling were carried out, I sampling (April, before starting the experimental trail), II sampling (May, after 1 month of feeding trail) and III sampling (June before breeding season). In the present study, a dose-dependent relationship between Vtg gene expression and zinc inclusion in the diet of threatened catfish, C. magur, was reported. Vtg gene expression increased in all groups from I sampling to II sampling but the highest Vtg gene expression was found in T1 group and the lowest in T3 group at II sampling. Vtg gene expression among the treatments differs significantly (P < 0.05) in each sampling. Accumulation of zinc was measured by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) in C. magur and it was reported that the significantly higher (P < 0.05) zinc was accumulated in the liver and ovary of T3 group as compared to other groups. The antioxidant enzyme activities (superoxide dismutase, SOD, catalase and GST) were also measured in different tissues (liver, gill and ovary) to evaluate the effect of extra-supplementation of zinc on the antioxidant status. In T3 group, SOD, catalase and GST activities were significantly higher than those in other groups. In the current study, serum glucose level was also measured and it was found in increasing trend with inclusion of zinc in the diet of C. magur. In the present study, it can be concluded that the zinc exhibits beneficial effect only up to 50 mg/kg. Thus, it is concluded that supplementation of zinc at 200 mg/kg or more disrupts Vtg gene expression and antioxidant status.



Similar content being viewed by others
Data availability
The data is available on request for any requirement and/or specific verification.
References
Jayaram KC (2006) Catfishes of India, 304–309 pp. Narendra Publishing house, New Delhi
Chakraborty A, Das S, Sarkar I, Dey A, Sinha M (2020) Seed production of endangered Indian Magur, Clarias magur (Hamilton, 1822) in low cost model with community participation. Int j fauna biol stud 7(3):17–20
Maurya PK, Shubham G, Majhi SK (2018) Factors posing threat to the endangered catfish Clarias magur (Hamilton 1822) and strategies for conservation. Environ Ecol 36(3):749–754
Vishwanath W (2010). Clarias magur. In: IUCN 2013. IUCN red list of threatened species. Version 2013.1. Available at: http://www.iucnredlist.org
Goswami B (2007) Magur (Clarias batrachus) seed production using low cost hatcheries: a participatory approach in Dakshin Dinajpur District of West Bengal. India Aquac Asia 12(3):14
Srivastava PP, Raizada S, Dayal R, Chowdhary S, Lakra WS, Yadav AK, Sharma P, Gupta J (2012) Breeding and larval rearing of Asian catfish, Clarias batrachus (Linnaeus, 1758) on live and artificial feed. J. Aquac Res Dev 3(4).DOI: https://doi.org/10.4172/2155-9546.1000134
Roy A, Mollah MFA (2009) Effects of different dietary levels of vitamin E on the ovarian development and breeding performances of Clarias batrachus (Linnaeus). J Bangladesh Agril Univ 7(1):183–191. https://doi.org/10.3329/jbau.v7i1.4983
Lubzens E, Lissauer L, Levavi-Sivan B, Avarre JC, Sammar M (2003) Carotenoid and retinoid transport to fish oocytes and eggs: what is the role of retinol binding protein? Mol Aspects Med 24(6):441–457. https://doi.org/10.1016/s0098-2997(03)00040-2
Avarre JC, Lubzens E, Babin PJ (2007) Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin II/I and vertebrate apolipoprotein. B. BMC Evol Biol 7 (3). Doi: https://doi.org/10.1186/1471-2148-7-3
Chatakondi NG, Kelly AM (2013) Oocyte diameter and plasma vitellogenin as predictive factors to identify potential channel catfish, Ictalurus punctatus, suitable for induced spawning. J World Aquac Soc 44(1):115–123. https://doi.org/10.1111/jwas.12001
Reading BJ, Sullivan CV, Schilling J (2011) Vitellogenesis in fishes. Encyclopedia of fish physiology: from genome to environment 1:635–646
Sandnes K, Ulgenes Y, OR, Utne F, (1984) The effect of ascorbic acid supllementation in brood fish seed on reproduction of rainbow trout Salmo guirdneri. Aquac 43:167–177. https://doi.org/10.1016/0044-8486(84)90019-X
Dhawan A, Kaur K (1997) Effect of zinc on maturation and breeding potential of Cyprinus carpio and Cirrhina mrigala. Int J Environ Stud 53(4):265–274. https://doi.org/10.1080/00207239708711130
Zhou XW, Zhu GN, Sun JH (2002) Effects of the interaction of heavy metals on the accumulation of copper in the tissues of the fish (Carassius auratus). J Zhejiang Univ Sci B 28(4):427–430 ((in Chinese))
Willis JN, Sunda WG (1984) Relative contributions of food and water in the accumulation of zinc by two species of marine fish. Mar Biol 80(3):273–279. https://doi.org/10.1007/bf00392822
Wei, W., Li, A., Li, D., 1999. Effect of dietary supplemented zinc on the growth and some biochemical parameters of juvenile flounder Paralichthys olioaceus . J. Ocean Uni. Qingdao 18, 60–66, in Chinese with English abstract .
Beaver LM, Nkrumah-Elie YM, Truong L, Barton CL, Knecht AL, Gonnerman GD, Wong CP, Tanguay RL, Ho E (2017) Adverse effects of parental zinc deficiency on metal homeostasis and embryonic development in a zebrafish model. J Nutr Biochem 43:78–87.doi/https://doi.org/10.1016/j.jnutbio.2017.02.006
Banks SD, Thomas P, Baer KN (1999) Seasonal variations in hepatic and ovarian zinc concentrations during the annual reproductive cycle in female channel catfish (Ictalurus punctatus). Comp Biochem Physiol C Toxicol Pharmacol Toxicol and Endocrinol 124(1):65–72. https://doi.org/10.1016/s0742-8413(99)00053-5
Shu Y, Gao Y, Sun H, Zou Z, Zhou Q, Zhang G (2009) Effects of zinc exposure on the reproduction of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Ecotox Environ Safe 72(8):2130–2136. https://doi.org/10.1016/j.ecoenv.2009.06.004
Tan LN, Feng L, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquacult Nutr 17(3):338–345. https://doi.org/10.1111/j.1365-2095.2010.00793.x
Akram Z, Fatima M, Shah SZH, Afzal M, Hussain SM, Hussain M, Khan ZI, Akram K (2019) Dietary zinc requirement of Labeo rohita juveniles fed practical diets. J Appl Anim Res 47(1):223–229. https://doi.org/10.1080/09712119.2019.1613238
Pierson KB (1981) Effects of chronic zinc exposure on the growth, sexual maturity, reproduction, and bioaccumulation of the guppy, Poecilia reticulata. Can. J. Fish. Aquat. Sci 38(1):23–31. https://doi.org/10.1139/f81-004
Buentello JA, Goff JB, Gatlin DM III (2009) Dietary zinc requirement of hybrid striped bass, Morone chrysops× Morone saxatilis, and bioavailability of two chemically different zinc compounds. J World Aquac Soc 40(5):687–694. https://doi.org/10.1111/j.1749-7345.2009.00288.x
Hook SE, Fisher NS (2002) Relating the reproductive toxicity of five ingested metals in calanoid copepods with sulfur affinity. Mar. Environ. Res. 53(161):174. https://doi.org/10.1016/s0141-1136(01)00118-0
Srivastava NK, Prakash S (2018) Effect of sublethal concentration of zinc sulphate on the serum biochemical parameters of freshwater cat fish, Clarias Batrachus (LINN). Indian J. Exp. Biol. 5 (2) DOI: https://doi.org/10.21088/ijb.2394.1391.5218.1
Huang F, Jiang M, Wen H, Wu F, Liu W, Tian J, Yang C (2015) Dietary zinc requirement of adult Nile tilapia (Oreochromis niloticus) fed semi-purified diets, and effects on tissue mineral composition and antioxidant responses Aquac439 :53–59.doi/https://doi.org/10.1016/j.aquaculture.2015.01.018
Cao L, Huang W, Liu J, Yin X, Dou S (2010) Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp. Biochem. Physiol. C 151(3):386–392. https://doi.org/10.1016/j.cbpc.2010.01.004
Tripathi BN, Mehta SK, Amar A, Gaur JP (2006) Oxidative stress in Scenedesmus sp. during short-andlong-term exposure to Cu2+ and Zn2. Chemosphere 62:538–544. https://doi.org/10.1016/j.chemosphere.2005.06.031
Lee SR (2018) Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev 2018:9156285. https://doi.org/10.1155/2018/9156285
Gupta G, Srivastava PP, Kumar M, Varghese T, Chanu TI, Gupta S, Ande MP Jana P (2021) The modulation effects of dietary zinc on reproductive performance and gonadotropins’(FSH and LH) expression in threatened Asian catfish, Clarias magur (Hamilton, 1822) brood fish Aquac Res 2021;00:1–12.doi: https://doi.org/10.1111/are.15077
AOAC (1995). Official methods of analysis (16th ed.). Association of official analytical chemists.
Aripin SA, Jintasataporn O, Yoonpundh R (2015) Effects of zinc amino acid in walking catfish (Clarias macrocephalus) female broodstock first sexual maturation. J Aquac Res Dev 6(7):1. https://doi.org/10.4172/2155-9546.1000347
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Bradford MM (1996) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Animal Biochemistry 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Takhara S, Hamilton HB, Neel JV, Kobara TY, Ogura Y, Nishimura ET (1960) Hypoctalasemia, a new genetic carrier state. J Clin Investig 39:610–619. https://doi.org/10.1172/JCI104075
Mishra HP, Frodovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and simple assay for SOD. J Biol Chem 247:3175. https://doi.org/10.1016/S0021-9258(19)45228-9
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-s-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Tang JX, Li JR, Liu ZL, Zhao H, Tao XM, Cheng ZS (2013) Effects of Zn2+ and Cu2+ on loach ovaries and ova development. Zool Res 34(E4−5):E135–E139
Korte JJ, Kahl MD, Jensen KM, Pasha MS, Parks LG, LeBlanc GA, Ankley GT (2000) Fathead minnow vitellogenin: complementary DNA sequence and messenger RNA and protein expression after 17β-estradiol. Environ Toxicol Chem 19:972–981. https://doi.org/10.1002/etc.5620190426
Xuefei L, Shao J, Zhou Q, Song M, Jiang G (2009) Circannual vitellogenin levels in Chinese loach (Misgurnus anguillicaudatus). Environmental Biology of Fishes 85, 23–29.155S.K. Garnayak et al. Aquac 392–395(2013):148–155
Pacoli CQ, Grizzle JM, Bradley JT (1990) Seasonal levels of serum vitellogenin and oocyte growth in the channel catfish Ictalurus punctatus. Aquac 90(3–4):353–367. https://doi.org/10.1016/0044-8486(90)90259-p
Kobayashi T, Pakarinen P, Torgersen J, Huhtaniemi I, Andersen O (2008) The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus) – 2. Differential follicle expression and asynchronous oogenesis. Gen Comp Endocrinol 156:595–602. https://doi.org/10.1016/j.ygcen.2008.02.010
Kaya S, Umucalilar HD, Haliloğlu S, İpek H (2001) Effect of dietary vitamin A and zinc on egg yield and some blood parameters of laying hens. Turk J Vet Anim Sci 25(5):763–769
Samuel MN, Pamela I, Ego OA, Samuel EO (2015) Effects of zinc oxide as forced-resting agent for layers on post-moult quality and nutritional value of eggs. J Chem Pharm Res 7(5):16–20
Guo YM, Yang R, Yuan J, Ward TL, Fakler TM (2006) Effect of Availa® Zn and zinc sulfate on egg zinc concentration, laying performance and egg quality. Program and Abstracts Bioavailability, pp.7–10.
Montorzi M, Falchuk KH, Vallee BL (1995) Vitellogenin and lipovitellin: zinc proteins of Xenopus laevis oocytes. Biochem 34(34):10851–10858. https://doi.org/10.1021/bi00034a018
Richards MP (1997) Trace mineral metabolism in the avian embryo. Poult Sci J 76(1):152–164. https://doi.org/10.1093/ps/76.1.152
de Alkimin, GD and Fracácio, R., 2020. Analysis of vitellogenin by histochemical method as an indicator of estrogenic effect in male Danio rerio exposed to metals. Environmental Science and Pollution Research, pp.1–5.do/i10.1007/s11356–020–08302–5
Thompson ED, Mayer GD, Glover CN, Capo T, Walsh PJ, Hogstrand C (2012) Zinc hyperaccumulation in squirrelfish (Holocentrus adscenscionis) and its role in embryo viability. PLoS ONE 7(10):46127. https://doi.org/10.1371/journal.pone.0046127
Nomizu T, Falchuk KH, Vallee BL (1993) Zinc, iron, and copper contents of Xenopus laevis oocytes and embryos. Mol Reprod Dev 36(4):419–423. https://doi.org/10.1002/mrd.1080360403
Schreck CB, Lorz HW (1978) Stress response of coho salmon (Oncorhynchus kisutch) elicited by cadmium and copper and potential use of cortisol as an indicator of stress. J Fish Res Board Can 35(8):1124–1129. https://doi.org/10.1139/f78-177
Alkahemal-Balawi HF, Ahmad Z, Al-Akel AS, Al-Misned F, Suliman EAM, Al-Ghanim KA (2011) Toxicity bioassay of lead acetate and effects of its sub-lethal exposure on growth, haematological parameters and reproduction in Clarias gariepinus. Afr J. Biotechnol 10(53):11039–11047. https://doi.org/10.5897/ajb11.1463
Pickering AD, Pottinger TG, Christie P (1982) Recovery of the brown trout, Salmo trutta L., from acute handling stress: a time‐course study. J. Fish Biol 20(2):229–244. https://doi.org/10.1111/j.1095-8649.1982.tb03923.x
Lee JW, Kim JE, Shin YJ, Ryu JS, Eom IC, Lee JS, Kim Y, Kim PJ, Choi KH, Lee BC (2014) Serum and ultrastructure responses of common carp (Cyprinus carpio L.) during long-term exposure to zinc oxide nanoparticles. Ecotox Environ Safe 104:9–17. https://doi.org/10.1016/j.ecoenv.2014.01.040
Hadi AA, Shokr AE, Alwan SF (2009) Effects of aluminum on the biochemical parameters of fresh water fish, Tilapia zillii. Journal of Science and its applications 3(1):33–41
Canli M (1995) Effect of Hg, Cr, Ni on glycogen reserves and protein levels in tissues of Cyprinus carpio. Turk J Zool 20:161–168
Basha PS, Rani AU (2003) Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicol Environ Safety 56(2):218–221
Barak P, Helmke PA (1993) The chemistry of zinc. In: Robson AD (ed) Zinc in soils and plants. Kluwer Academic Publishers, Dordrecht, pp 90–106
Gupta G, Chatterjee A, Kumar M, Sardar P, Varghese T, Srivastava PP, Gupta S (2020) Efficacy of single and multiple doses of fenbendazole against gill parasites (Dactylogyrus sp.) of Labeo rohita (Hamilton, 1822) and its physio‐metabolic effects on the fish. Aquac Res 51(3):1190–1199
Gupta G, Kumar M, Sardar P, Varghese T, Srivastava PP, Gupta S (2021) Pharmacokinetics and physio-metabolic response of single and multiple dose of fenbendazole in Labeo rohita (Hamilton, 1822) fingerlings. Aquac Res 52(1):260–272. https://doi.org/10.1111/are.14889
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10(1):49
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 54(4):287–293. https://doi.org/10.1016/j.ajme.2017.09.001
Miessler GL, Tarr DA (2010) Inorg Chem 2010:644–647
Richardson J, Thomas KA, Rubin BH, Richardson DC (1975) Crystal structure of bovine Cu,Zn superoxide dismutase at 3 Å resolution: chain tracing and metal ligands. Proc Natl Acad Sci USA 72(4):1349–1353
Meiler K, Cleveland B, Radler L, Kumar V (2020) Oxidative stress-related gene expression in diploid and triploid rainbow trout (Oncorhynchus mykiss) fed diets with organic and inorganic zinc. Aquac 533:736149. https://doi.org/10.1016/j.aquaculture.2020.736149
Jiang M, Wu F, Huang F, Wen H, Liu W, Tian J, Yang CG, Wang WM (2016) Effects of dietary Zn on growth performance, antioxidant responses, and sperm motility of adult blunt snout bream. Megalobrama amblycephala Aquac 464:121–128
Banni M, Chouchene L, Said K, Kerkeni A, Messaoudi I (2011) Mechanisms underlying the protective effect of zinc and selenium against cadmium-induced oxidative stress in zebrafish Danio rerio. Biometals 24:981–992. https://doi.org/10.1007/s10534-011-9456-z
Feng L, Tan LN, Liu Y, Jiang J, Jiang WD, Hu K, Li SH, Zhou XQ (2011) Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defence of juvenile Jian carp (Cyprinus carpio var. Jian). Aquacult Nutr 17:E875–E882
Guo J, He L, Li T, Yin J, Yin Y, Guan G (2020) Antioxidant and anti-inflammatory effects of different zinc sources on diquat-induced oxidant stress in a piglet model. BioMed Res. Int, 2020.
Chung MJ, Walker PA, Hogstrand C (2004) Metal physiology and biochemistry in fish cells: from toxicity to protection by zinc. Comp Biochem Physiol C 100:137–147
Srikanth K, Pereira E, Duarte AC, Ahmad I (2013) Glutathione and its dependent enzymes’ modulatory responses to toxic metals and metalloids in fish—a review. Environ Sci Pollut Res 20(4):2133–2149
Funding
The funding was for student research and was provided by the institute itself. No external funding was available.
Author information
Authors and Affiliations
Contributions
Gyandeep Gupta: conceptualisation and data curation; Prem Prakash Srivastava: conceptualisation, formal analyses, sources and software; Gyandeep Gupta, Prem Prakash Srivastava: roles in writing-original draft. Tincy Varghese: writing-review and editing and supervision; Thongam I. Chanu, Subodh Gupta, Muralidhar P. Ande, Gopal Krishna: review and editing; Prashana Jana: help in sampling, review and editing.
Corresponding author
Ethics declarations
Ethics Approval
The ICAR-CIFE research work on fish was approved by the ICAR-CIFE Research Ethics Review Committee.
Consent to Participate
All the authors participated as at appropriate to the work.
Consent for Publication
Authors permit the Editor to publish this paper in the journal, BTER.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, G., Srivastava, P.P., Gangwar, M. et al. Extra-Fortification of Zinc Upsets Vitellogenin Gene Expression and Antioxidant Status in Female of Clarias magur brooders. Biol Trace Elem Res 200, 1861–1871 (2022). https://doi.org/10.1007/s12011-021-02793-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02793-0