Abstract
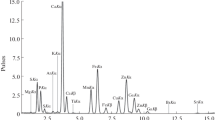
A total of 158 serum samples of newly diagnosed type 2 diabetes patients and control subjects were analyzed using Synchrotron Radiation X-ray Fluorescence (SRXRF) technique. The microprobe XRF beam line-16 of Indus-2 synchrotron radiation facility at Raja Ramanna Centre for Advanced Technology (RRCAT), Indore, India, was used to identify and quantify the elements K, Ca, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Br, Rb, Sr, and Pb. A significant decrease in the mean concentrations of K, Ca, Ti, Cr, Mn, Ni, Zn, and As and an increase in the concentrations of V, Fe, Co, Cu, Se, and Pb were observed in the serum samples of the patient group when compared to the control group. It is hypothesized that the observed alterations in the elemental concentrations might have led to ineffective uptake of insulin and have interfered with glucose homeostasis by either directly or indirectly causing oxidative stress.



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Insulin and associated devices: access for everybody (2020) WHO stakeholder workshop, 21 and 23–25
Definition, diagnosis, and classification of diabetes mellitus and its complications (1999) Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus, World Health Organization, Geneva, Switzerland
Glechner A, Keuchel L, Affengruber L, Titscher V, Sommer I, Matyas N, Gartlehner G (2018) Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim Care Diabetes 12(5):393–408. https://doi.org/10.1016/j.pcd.2018.07.003
Hu FB (2011) Globalization of Diabetes role of diet lifestyle and genes. Diabetes care 34(6):1249–57. https://doi.org/10.2337/dc11-0442
Capurso A, Crepaldi G, Capurso C (2020) The Mediterranean diet: a pathway to successful aging. Aging Clin Exp Res 32:1187–1188. https://doi.org/10.1007/s40520-019-01160-3
Hu C, Jia W (2018) Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes 67(1):3–11. https://doi.org/10.2337/dbi17-0013
International Diabetes Federation (2019) Diabetes Atlas 9th edition
Augustsson AL, Uddh-Soderberg TE, Hogmalm KJ, Filipsson ME (2015) Metal uptake by homegrown vegetables – the relative importance in human health risk assessments at contaminated sites. Environ Res 138:181–190. https://doi.org/10.1016/j.envres.2015.01.020
Pena-Fernandez A, Gonzalez-Munoz MJ, Lobo-Bedmar MC (2014) Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ Int 72:176–185. https://doi.org/10.1016/j.envint.2014.04.007
Bocca B, Pino A, Alimonti A, Forte G (2014) Toxic metals contained in cosmetics: a status report. Regul Toxicol Pharmacol 68(3):447–467. https://doi.org/10.1016/j.yrtph.2014.02.003
Chowdhury S, Mazumder MA, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569:476–488. https://doi.org/10.1016/j.scitotenv.2016.06.166
Cancarini A, Fostinelli J, Napoli L, Gilberti ME, Apostoli P, Semeraro F (2017) Trace elements and diabetes: assessment of levels in tears and serum. Exp Eye res 154:47–52. https://doi.org/10.1016/j.exer.2016.10.020
Durak R, Gülen Y, Kurudirek M, Kaçal M, Çapoğlu İ (2010) Determination of trace element levels in human blood serum from patients with type II diabetes using WDXRF technique: a comparative study. J Xray Sci Technol 18(2):111–120. https://doi.org/10.3233/XST-2010-0247
Zhang H, Yan C, Yang Z, Zhang W, Niu Y, Li X, Qin L, Su Q (2017) Alterations of serum trace elements in patients with type 2 diabetes. J Trace Elem Med Biol 40:91–96. https://doi.org/10.1016/j.jtemb.2016.12.017
Wolide AD, Zawdie B, Alemayehu T, Tadesse S (2017) Association of trace metal elements with lipid profiles in type 2 diabetes mellitus patients: A cross sectional study. BMC Endocr Disord 17:64. https://doi.org/10.1186/s12902-017-0217-z
Elayaperumal M, Vedachalam Y, Loganathan D, Kumaravelu TA, Ganesh SA, Kennedy J, (2020) Ion beam analysis of proton induced X-ray emission (PIXE) techniques for elemental investigation of young stage neem leaf of southern India, Tamil Nadu. Biol Trace Elem Res https://doi.org/10.1007/s12011-020-02443-x
Naidu BG, Srikanth S, Naga Raju GJ, Sarita P (2020) PIXE analysis of blood serum of breast cancer patients undergoing successive chemotherapy. J Radioanal Nucl Chem 323:1307–1316. https://doi.org/10.1007/s10967-019-06988-7
Gowri Naidu B, Sarita P, Naga Raju GJ, Tiwari MK (2019) Multivariate analysis of trace elemental data obtained from blood serum of breast cancer patients using SRXRF. Results in Physics 12:673–680. https://doi.org/10.1016/j.rinp.2018.12.020
Naga Raju GJ, Sarita P, Murthy KSR (2017) (2017) Comparative trace elemental analysis of cancerous and non-cancerous tissues of rectal cancer patients using PIXE. Nucl Instrum Meth B 404:146–149. https://doi.org/10.1016/j.nimb.2017.01.060
Sarita P, Naga Raju GJ, Pradeep AS, RautrayTapash R, Seetharami Reddy B, Bhuloka Reddy S, Vijayan V (2012) Analysis of trace elements in blood sera of breast cancer patients by particle induced X-ray emission. J Radioanal Nucl Chem 294:355–361. https://doi.org/10.1007/s10967-011-1505-0
Manikandan E, Prithiviraj B, Kavitha G, Magudapathy P, Rajamannan B (2011) 2 MeV-PIXE technique for coastal material analysis. Int J of PIXE 21(34):75–86. https://doi.org/10.1142/S0129083511002215
Gowrishankar R, Kumar M, Menon V, Divi SM, Saravanan M, Magudapathy P, Panigrahi BK, Nair KGM, Venkataramaniah K (2010) Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae) and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Elem Res 133:357–363. https://doi.org/10.1007/s12011-009-8439-1
Van Grieken RE, Markowicz AA (1993) Handbook of X-ray spectrometry, New York
Sole VA, Papillon E, Cotte M, Walter Ph, Susini J (2007) Multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta, Part B 62(1):63–68. https://doi.org/10.1016/j.sab.2006.12.002
Pohl HR, Wheeler JS, Murray HE (2013) Sodium and potassium in health and disease. Interrelations between essential metal ions and human diseases. 29–47
Rodan AR (2017) Potassium: friend or foe? Pediatr Nephrol 32(7):1109–1121. https://doi.org/10.1007/s00467-016-3411-8
Pollare T, Lithell H, Berne C (1989) A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N Engl J Med 321:868–873. https://doi.org/10.1056/NEJM198909283211305
Helderman JH, Elahi D, Anderson DK, Raizes GS, Tobin JD, Shocken D, Andres R (1983) Prevention of the glucose intolerance of thiazide diuretics by maintenance of body potassium. Diabetes 32:106–111. https://doi.org/10.1007/978-3-642-71487-0_10
Xu Q, Xu F, Fan L, Xiong L, Li H, Cao S et al (2014) Serum Potassium Levels and Its Variability in Incident Peritoneal Dialysis Patients: Associations with Mortality. PLoS ONE 9(1):e86750. https://doi.org/10.1371/journal.pone.0086750
Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D (2009) Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med 169(17):1587–1594. https://doi.org/10.1001/archinternmed.2009.284
Ozcan L, Tabas I (2016) Calcium signaling and ER stress in insulin resistance and atherosclerosis. J Intern Med 280:457–464. https://doi.org/10.1111/joim.12562
Sun G, Vasdev S, Martin G, Gadag V, Zhang H (2005) Altered Calcium Homeostasis Is Correlated With Abnormalities of Fasting Serum Glucose, Insulin Resistance, and -Cell Function in the Newfoundland Population. Diabetes 54:3336–3339. https://doi.org/10.2337/diabetes.54.11.3336
Mooradian AD, Morely JE (1987) Micronutrient status in diabetes mellitus. Am J Clin Nutr 45:877–895. https://doi.org/10.1093/ajcn/45.5.877
Gurson CT, Saner G (1971) Effect of chromium on glucose utilization in marasmic protein–calorie malnutrition. Am J Clin Nutr 24:1313–1319. https://doi.org/10.1093/ajcn/24.11.1313
Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK, Wiederkehr A, Wollheim CB, Lee IK, Park KS (2017) Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med 49:e291–e291. https://doi.org/10.1038/emm.2016.157
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029. https://doi.org/10.1210/jc.2007-0298
Jacqmain M, Doucet E, Despres JP, Bouchard C, Tremblay A (2003) Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am J Clin Nutr 77:1448–1452. https://doi.org/10.1093/ajcn/77.6.1448
Aune D, Norat T, Romundstad PR, Vatten LJ (2013) Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 98:1066–1083. https://doi.org/10.3945/ajcn.113.059030
Gijsbers L, Ding EL, Malik VS, De Goede J, Geleijnse JM, Soedamah-Muthu SS (2016) Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am J Clin Nutr 103:1111–1124. https://doi.org/10.3945/ajcn.115.123216
Rooney MR, Pankow JS, Shalamar D, Sibley SD, Selvin E, Reis JP, Michos ED, Lutsey PL (2016) Serum calcium and incident type 2 diabetes the Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr 104(4):1023–1029. https://doi.org/10.3945/ajcn.115.130021
Chen C, Jiang W, Zhong N, Wu J, Jiang H, Du J, Li Y, Ma X, Hashimoto ZM, K, et al (2014) Impaired processing speed and attention in first-episode drug naive schizophrenia with deficit syndrome. Schizophr Res 159:478–484. https://doi.org/10.1016/j.schres.2014.09.005
Kawasaki N, Matsui K, Ito M, Nakamura T, Yoshimura T, Ushijima H, Maeyama M (1985) Effect of calcium supplementation on the vascular sensitivity to angiotensin II in pregnant women. Am J Obstet Gynecol 153:576–582. https://doi.org/10.1016/0002-9378(85)90482-x
Kalman DS (2003) Chromium picolinate and type 2 diabetes. Am J Clin Nutr 78(1):192. https://doi.org/10.1093/ajcn/78.1.192
Anderson RA, Polansky MM, Bryden NA, Bhathena SJ, Canary J (1987) Effects of supplemental Chromium on patients with symptoms of reactive hypoglycemia. Metabolism 36:351–355. https://doi.org/10.1016/0026-0495(87)90206-x
Anderson RA (1998) Chromium, glucose intolerance and diabetes. J Am Coll Nutr 17(6):548–555. https://doi.org/10.1080/07315724.1998.10718802
Anderson RA (1977) Nutritional factors influencing the glucose/insulin system: Chromium. J Am Coll Nutr 16:404–410. https://doi.org/10.1080/07315724.1997.10718705
Farrokhian A, Mahmoodian M, Bahmani F, Amirani E, Shafabakhsh R, Asemi Z (2020) The Influences of Chromium Supplementation on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Biol Trace Elem Res 194:313–320. https://doi.org/10.1007/s12011-019-01783-7
Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, Glinsmann W (1983) Chromium supplementation of human subjects: Effects on glucose, insulin, and lipid variables. Metabolism 32:894–899. https://doi.org/10.2337/dc06-0996
Chen ZC, Ma JF (2015) Improving Nitrogen Use Efficiency in Rice through Enhancing Root Nitrate Uptake Mediated by a Nitrate Transporter, NRT1.1B. J Genet Genom 42:463–465. https://doi.org/10.1016/j.jgg.2015.08.003
Balk E, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG (2007) Effect of Chromium Supplementation on Glucose Metabolism and Lipids: A systematic review of randomized controlled trials. Diabetes Care 30:2154–2163. https://doi.org/10.2337/dc06-0996
Kazi TG, Afridi HI, Kazi N, Jamali MK, ArainMB JN, Kandhro GA (2008) Copper, Chromium, Manganese, Iron, Nickel, and Zinc Levels in Biological Samples of Diabetes Mellitus Patients. Biol Trace Element Res 122:1–18. https://doi.org/10.1007/s12011-007-8062-y
Adam KM, Ibrahim HAE (2015) Level of Serum Chromium in Sudanese Type 1 and Type 2 Diabetes Mellitus Patients. Am J Biochem 5(4):7673–7676. https://doi.org/10.5923/j.ajb.20150504.01
Prasad AS (2014) Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol 28(4):357–363. https://doi.org/10.3945/an.112.003210
Li YV (2013) Zinc and insulin in pancreatic beta-cells. Endocrine 45:178–189. https://doi.org/10.1007/s12020-013-0032-x
Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ (1983) Abnormal zinc metabolism in type II diabetes mellitus. Am J Med 75:273–277. https://doi.org/10.1016/0002-9343(83)91205-6
Abou-Seif MA, Youssef AA (2004) Evaluation of some biochemical changes in diabetic patients Clin ChimActa 346:161–170. https://doi.org/10.1016/j.cccn.2004.03.030
Xu J, Zhou Q, Liu G, Tan Y, Cai L (2013) Analysis of Serum and Urinal Copper and Zinc in Chinese Northeast Population with the Prediabetes or Diabetes with and without Complications. Oxid Med Cell Longev 2013:635214. https://doi.org/10.1155/2013/635214
Anil Kumar VSPD, Jaiprabhu J, Krishnan R (2014) Serum copper and zinc levels significance in type 2 diabetic patients. J Med Sci Tech 3:79–81. https://doi.org/10.5812/ijem.33273
Kloubert V, Rink L (2015) Zinc as a micronutrient and its preventive role of oxidative damage in cells. Food Funct 6:3195–3204. https://doi.org/10.1039/c5fo00630a
Fernandez-Real JM, McClain D, Manco M (2015) Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care 38:2169–2176. https://doi.org/10.2337/dc14-3082
Fernandez-Real JM, López-Bermejo A, Ricart-Engel W (2002) Cross-talk between iron metabolism and diabetes. Diabetes 51:2348–2354. https://doi.org/10.2337/diabetes.51.8.2348
Lao TT, Chan PL, Tam KF (2001) Gestational diabetes mellitus in the last trimester—A feature of maternal iron excess? Diabetic Med J Br Diabet Assoc 18:218–223. https://doi.org/10.1046/j.1464-5491.2001.00453.x
Liu J, Li Q, Yang Y, Ma L (2020) Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. Journal of diabetes investigation 11(4):946–955. https://doi.org/10.1111/jdi.13216
Li SY, Wang F, Lu XT, Zhong RH, Long JA, Fang AP, Zhu HL (2020) Dietary iron intake and the risk of type 2 diabetes mellitus in middle-aged and older adults in urban China: A Prospective Cohort Study. Br J Nutr 14:1–22. https://doi.org/10.1017/S0007114520005048
Krisai P, Leib S, Aeschbacher S, Kofler T, Assadian M, Maseli A, Todd J, Estis J, Risch M, Risch L et al (2016) Relationships of iron metabolism with insulin resistance and glucose levels in young and healthy adults. Eur J Intern Med 32:31–37. https://doi.org/10.1016/j.ejim.2016.03.017
Bao W, Rong Y, Rong S et al (2012) Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med 10(1):1–3. https://doi.org/10.1186/1741-7015-10-119
Liu J, Li Q, Yang Y, Ma L (2020) Iron metabolism and type 2 diabetes mellitus: A meta-analysis and systematic review. J Diabetes Investig 11(4):946–955. https://doi.org/10.1111/jdi.13216
Simcox JA, McClain DA (2013) Iron and diabetes risk Cell Metab 17:329–341. https://doi.org/10.1016/j.cmet.2013.02.007
Lal M, Beena SK, Shetty V, Rao GM (2013) Influence of modified levels of plasma magnesium, copper, zinc and iron levels on thiols and protein status in diabetes mellitus and diabetic retinopathy. IJAPBS 2:67–72. http://eprints.manipal.edu/id/eprint/79723
Kazi TG, Afridi HI, Kazi N, Jamali MK, Arain MB, Jalbani N, Kandhro GA (2008) Copper, chromium, manganese, iron, nickel, and zinc levels in biological samples of diabetes mellitus patients. Biol Trace Elem Res 122:1–18. https://doi.org/10.1007/s12011-007-8062-y
Serdar MA, Bakir F, Haşimi A, Çelik T, Akin O, Kenar L, Aykut O, Yildirimkaya M (2009) Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J Diabetes Dev Ctries 29:35–40. https://doi.org/10.4103/0973-3930.50713
Olaniyan OO, Awonuga MAM, Ajetunmobi AF, Adeleke IA, Fagbolade OJ, Olabiyi KO, Oyekanmi BA, Osadolor HB (2012) Serum copper and zinc levels in Nigerian type 2 diabetic patients. Afr J Diabetes Med 20:36–38
Liu A, Xu P, Gong C, Zhu Y, Zhang H, Nie W, Zhou X, Liang X, Xu Y, Huang C, Liu XL (2020) High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: A health examination center based cross-sectional study. J Trace Elem Med Biol 59:126470. https://doi.org/10.1016/j.jtemb.2020.126470
Zargar AH, Shah NA, Masoodi SR, Laway BA, Dar FA, Khan AR, Sofi FA, Wani AI (1998) Copper, zinc and magnesium levels in non-insulin dependent diabetes mellitus. Postgrad Med J 74:665–668. https://doi.org/10.1136/pgmj.74.877.665
Prousek J (2007) Fenton chemistry in biology and medicine. Pure Appl Chem 79:2325–2338. https://doi.org/10.1351/pac200779122325
Liochev SI, Fridovich I (2002) The Haber-Weiss cycle-70 years later: an alternative view. Redox Rep 7:55–57. https://doi.org/10.1179/135100002125000190
Masad A, Hayes L, Tabner BJ et al (2007) Copper-mediated formation of hydrogen peroxide from the amylin peptide: a novel mechanism for degeneration of islet cells in type-2 diabetes mellitus? FEBS Lett 581(18):3489–3493. https://doi.org/10.1016/j.febslet.2007.06.061
Barbusinski K (2009) Fenton reaction-controversy concerning the chemistry. Ecol Chem Eng 16:347–358
Harding JL, Pavkov ME, Magliano DJ et al (2019) Global trends in diabetes complications: a review of current evidence. Diabetologia 62:3–16. https://doi.org/10.1007/s00125-018-4711-2
Mooradian AD, Morely JE (1987) Micronutrient status in diabetes mellitus. Am J Clin Nutr 45:877–895. https://doi.org/10.1093/ajcn/45.5.877
Davis GK, Mertz W (1987) Copper. In: Mertz W (ed) Trace Elements in Human and Animal Nutrition, vol 1. Academic Press, Orlando, p 301
Basaki M, Saeb M, Nazifi S, Shamsaei HA (2012) Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res 148:161–164. https://doi.org/10.1007/s12011-012-9360-6
Akinloye O, Ogunleye K, Oguntibeju OO (2010) Cadmium, lead, arsenic and selenium levels in patients with type 2 diabetes mellitus. Afr J Biotech 9:5189–5195
Kljai K, Runje R (2001) Selenium and glycogen levels in diabetic patients. Biol Trace Elem Res 83:223–229. https://doi.org/10.1385/BTER:83:3:223
Tabar MB (2012) Determination of Serum Selenium in Patients with Type II Diabetes Mellitus. Middle-East J Sci Res 12:433–435. https://doi.org/10.5829/idosi.mejsr.2012.12.4.2058
Asare GA, Osae S, Nortey ENN, Yambire FK, Amedonu E, Doku D, Annan Y (2013) Evaluation of serum metallothionein-1, selenium, zinc, and copper in Ghanaian type 2 diabetes mellitus patients. Int J Diabetes Dev Ctries 33:86–95. https://doi.org/10.1007/s13410-013-0111-9
Ekmekcioglu C, Prohaska C, Pomazal K, Steffan I, Schernthaner G, Marktl W (2001) Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol Trace Elem Res 79:205–219. https://doi.org/10.1385/BTER:79:3:205
Wang WC, Makela AL, Nanto V, Makela P (1995) Serum selenium levels in diabetic children. Biol Trace Element Res 47:355–364. https://doi.org/10.1007/BF02790138
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 1790:1478–1485. https://doi.org/10.1016/j.bbagen.2009.02.014
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727. https://doi.org/10.1074/jbc.R800045200
Yang Z, Xie Y, Chen J, Zhang D, Yang C, Li M (2010) High selenium may be a risk factor of adolescent idiopathic scoliosis. Med Hypotheses 75:126–127. https://doi.org/10.1016/j.mehy.2010.02.006
Vinceti M, Maraldi T, Bergomi M, Malagoli C (2009) Risk of chronic low-dose selenium overexposure in humans: insights from epidemiology and biochemistry. Rev Environ Health 24:231–248. https://doi.org/10.1515/reveh.2009.24.3.231
Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, Baig JA, Sarfraz RA (2008) Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clinical Pr 80:280–288. https://doi.org/10.1016/j.diabres.2007.12.021
Hermes-Lima M, Pereira B, Bechara EJ (1991) Are free radicals involved in lead poisoning? Xenobiotica 8:1085–1090. https://doi.org/10.3109/00498259109039548
Bechara EJ, Medeiros MH, Monteiro HP, Hermes-Lima M, Pereira B, Demasi M (1993) A free radical hypothesis of lead poisoning and inborn porphyrias associated with 5- aminolevulinic acid overload. Quim Nova 16:385–392
Acknowledgements
One of the authors, P. Sarita, acknowledges the financial support rendered by University Grants Commission -Department of Atomic Energy Consortium for Scientific Research, Indore Centre, India, to carry out this work in the form of a Collaborative Research Scheme (CSR-IC-BL-60/CRS-177/2016-17/841 dated 28th October 2016). The authors thank the authorities and staff of Indus-2 synchrotron radiation utilization facility at Raja Ramanna Centre for Advanced Technology (RRCAT), Indore, India, for the providing technical assistance. The authors appreciate the support of the medical personnel of Endocrinology Department, Andhra Medical College, Visakhapatnam, during sample collection.
Funding
This research is supported by University Grants Commission – Department of Atomic Energy Consortium for Scientific Research, Indore Centre, India, in the form a sanctioned Collaborative Research Scheme (CRS) (CSR-IC-BL-60/CRS-177/2016–17/841 dated 28th October 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by Ethics Committee of Andhra Medical College, Visakhapatnam, India. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rao, N.S., Raju, G.J.N., Tiwari, M.K. et al. Serum Elemental Analysis of Type 2 Diabetes Patients Using SRXRF. Biol Trace Elem Res 200, 1485–1494 (2022). https://doi.org/10.1007/s12011-021-02762-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02762-7