Abstract
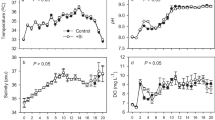
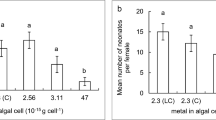
Trace element supplementation to the freshwater environment can influence the plankton density and species diversity, contributing to the nutrition of aquaculture species, especially during the juvenile stage. An experiment was conducted under laboratory conditions to evaluate the effects of supplementing different mixtures of manganese, silica and phosphorus on the plankton density and species diversity and their impact on cultured juvenile marron (Cherax cainii, Austin and Ryan, 2002). Manganese, silica and phosphorus in concentrations of 0.0024, 0.41, 0.05 mg*L−1; 0.0041, 0.82, 0.12 mg*L−1; and 0.0058, 1.26, 0.25 mg*L−1 respectively termed as low, medium and high were supplemented to tank water containing a phytoplankton density of 3.77 ± 0.16 × 106 cells*L−1 and 292.9 ± 17.6 individuals*L−1 of zooplankton, and plankton growth was observed every 24 h for 6 days. Afterwards, a 3-month trial was conducted studying the effects of these trace element concentrations and resulting plankton densities on marron growth, survival, moulting, gut microbiota and health indices. Silica supplementation at high concentration increased the diatom abundance, silica and phosphorus supplementation at higher concentration that resulted in a significant increase in plankton density and species diversity, leading to improved marron health indices than the control and the tanks receiving a low concentration. Marron-specific growth rate, weight gain and dissolved copper concentration in haemolymph were significantly higher in tanks with higher supplementation and higher plankton density. Marron survival, moult interval and total haemocyte count were not affected by the supplementation. Marron gut microbiota at higher trace element concentration supplementation showed a significant increase in abundance of phosphate solubilizing bacteria.



Similar content being viewed by others
Data Availability
The experimental data will be provided on request and the raw data for marron gut microbiota in FASTQ files has been deposited to National Centre for Biotechnology Information (NCBI) BioProject under the accession number PRJNA682157.
Code Availability
Not applicable.
References
Machin D, Dearden M, Lacey P (2008) Marron aquaculture strategic extension campaign- an interim report. The Regional institure online publishing. http://www.regional.org.au/au/apen/2003/non_refereed/080machind.htm. Accessed 24 March 2020
Alonso AD (2009) Marron farming and environmental sustainability: Western Australia’s case. Environmentalist 29:388–397. https://doi.org/10.1007/s10669-008-9211-3
Abdel-Tawwab M, Abdelghany AE, El-Ayouty YM, El-Essawy A-FA (2002) Effect of different doses of inorganic fertilizer on water quality, primary productivity and production of Nile tilapia (Oreochromis niloticus) in earthen ponds. Qatar Univ Sci J 22:81–95 https://hdl.handle.net/10576/10328
Adhikari S (2003) Fertilization, soil and water quality management in small-scale ponds. Gher Revolution 8:1–52
Drenner RW, Threlkeld ST, Smith JD, Mummert JR, Cantrell PA (1989) Interdependence of phosphorus, fish, and site effects on phytoplankton biomass and zooplankton. Limnol Oceanogr 34:1315–1321
Tew KS, Conroy JD, Culver DA (2006) Effects of lowered inorganic phosphorus fertilization rates on pond production of percid fingerlings. Aquaculture 255:436–446. https://doi.org/10.1016/j.aquaculture.2006.01.003
Azim M, Little D (2006) Intensifying aquaculture production through new approaches to manipulating natural food. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 1:1–23. https://doi.org/10.1079/PAVSNNR20061062
Boyd CE (2018) Aquaculture pond fertilization. CAB Rev 13:1–12. https://doi.org/10.1079/PAVSNNR201813002
Boyd CE (2014) Silicon, diatoms in aquaculture. Glob Aquac Advocate 17:38–39
Goldman C (2010) Micronutrient elements (Co, Mo, Mn, Zn, Cu). In: Likens GE (ed) Biogeochemistry of inland waters. Academic Press, San Diego, pp 378–382
Li Y, Wang HZ, Liang XM, Yu Q, Xiao XC, Shao JC, Wang HJ (2017) Total phytoplankton abundance is determined by phosphorus input: evidence from an 18-month fertilization experiment in four subtropical ponds. Can J Fish Aquat Sci 74:1454–1461. https://doi.org/10.1139/cjfas-2016-0057
Nwankwegu AS, Li Y, Huang Y, Wei J, Norgbey E, Lai Q, Sarpong L, Wang K, Ji D, Yang Z (2020) Nutrient addition bioassay and phytoplankton community structure monitored during autumn in xiangxi bay of three gorges reservoir, china. Chemosphere 247:1–11. https://doi.org/10.1016/j.chemosphere.2020.125960
Pace ML, Lovett G (2013) Primary production: the foundation of ecosystems. In: Weathers KC, Strayer DL, Likens GE (eds) Fundamentals of ecosystem science, 2nd edn. Elsevier Academic Press, London, pp 27–51
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2020) Effects of seasonal variations and pond age on trace elements and their correlations with plankton productivity in commercial freshwater crayfish (Cherax cainii austin, 2002) earthen ponds. Aquac Res 51:1913–1922. https://doi.org/10.1111/are.14542
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, San Diego, pp 239–328
Duffy RE, Godwin I, Nolan J, Purvis I (2011) The contribution of naturally occurring food items to the diet of Cherax destructor when fed formulated diets of differing protein levels. Aquaculture 313:107–114. https://doi.org/10.1016/j.aquaculture.2010.11.040
Jones P, Austin C, Mitchell B (1995) Growth and survival of juvenile Cherax albidus Clark cultured intensively on natural and formulated diets. Freshw Crayfish 10:480–493
Gonzalez A, Celada JD, Carral JM, Saez-Royuela M, Garcia V, Gonzalez R (2012) Effects of live artemia nauplii supplementation for different periods on survival and growth of juvenile signal crayfish Pacifastacus leniusculus in the first six months of intensive culture. N Am J Aquac 74:34–38. https://doi.org/10.1080/15222055.2011.649392
Sáez-Royuela M, Carral J, Celada J, Pérez J, González A (2007) Live feed as supplement from the onset of external feeding of juvenile signal crayfish (Pacifastacus leniusculus Dana. Astacidae) under controlled conditions. Aquaculture 269:321–327. https://doi.org/10.1016/j.aquaculture.2007.04.053
Sierp MT, Qin JG (2001) Effects of fertiliser and crayfish on plankton and nutrient dynamics in hardwater ponds. Hydrobiologia 462:1–7. https://doi.org/10.1023/A:1013184502119
Lambert CW (2019) Long-term effects of elevated manganese on Procambarus clarkii behavior. Dissertation, Marshall University
Jussila J, Henttonen P, Huner JV (1995) Calcium, magnesium and manganese content of noble crayfish (Astacus astacus (L.)) branchial carapace and its relationship to water and sediment mineral content of two ponds and one lake in Central Finland. Freshw Crayfish 10:230–238
Hossain MM, Huang H, Yuan Y, Wan T, Jiang C, Dai Z, Xiong S, Cao M, Tu S (2021) Silicone stressed response of crayfish (Procambarus clarkii) in antioxidant enzyme activity and related gene expression. Environ Pollut 274:1–11. https://doi.org/10.1016/j.envpol.2020.115836
Ackefors H (1996) The development of crayfish culture in Sweden during the last decade. Freshw Crayfish 11:627–654
Oweson CA, Baden SP, Hernroth BE (2006) Manganese induced apoptosis in haematopoietic cells of Nephrops norvegicus (L.). Aquat Toxicol 77:322–328. https://doi.org/10.1016/j.aquatox.2006.01.008
Kayath CA, Ibala Zamba A, Goma-Tchimbakala J, Mamonékéné V, Mombo Makanga GM, Lebonguy AA, Nguimbi E (2019) Microbiota landscape of gut system of guppy fish (Poecilia reticulata) plays an outstanding role in adaptation mechanisms. Int J Microbiol 2019:1–10. https://doi.org/10.1155/2019/3590584
Morrissy N (1990) Optimum and favourable temperatures for growth of Cherax tenuimanus (Smith 1912)(Decapoda: Parastoacidae). Mar Freshw Res 41:735–746
Ingram BA, Shiel RJ, Hawking JH (1997) Aquatic life in freshwater ponds: a guide to the identification and ecology of life in aquaculture ponds and farm dams in south eastern Australia. Co-operative Research Centre for Freshwater Ecology Albury, NSW, Australia
Canter-Lund H, Lund J (1995) Freshwater algae: their microscopic world explored. Biopress Ltd., Bristol, pp 1–360
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2021) Temporal variations and pond age effect on plankton communities in semi-intensive freshwater marron (Cherax cainii, Austin and Ryan, 2002) earthen aquaculture ponds in Western Australia. Saudi J Biol Sci 28:1392–1400. https://doi.org/10.1016/j.sjbs.2020.11.075
APHA (2012) (American Public Health Association) Standard methods for the examination of water and wastewater.
Baden SP, Neil DM (1998) Accumulation of manganese in the haemolymph, nerve and muscle tissue of Nephrops norvegicus (L.) and its effect on neuromuscular performance. Com Biochem Physiol Part A Mol Integr Physiol 119:351–359. https://doi.org/10.1016/S1095-6433(97)00437-6
Nugroho RA, Fotedar R (2013) Dietary organic selenium improves growth, survival and resistance to Vibrio mimicus in cultured marron, Cherax cainii (Austin, 2002). Fish Shellfish Immunol 35:79–85. https://doi.org/10.1016/j.fsi.2013.04.011
Fotedar R (1998) Nutrition of marron, Cherax tenuimanus (Smith) under different culture environments: a comparative study. Dissertation, Curtin University
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Joshi N, Fass J (2011) Sickle-a windowed adaptive trimming tool for FASTQ files using quality. https://github.com/najoshi/sickle
Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU (2016) MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinf 17:1–6. https://doi.org/10.1186/s12859-016-1358-1
Albanese D, Fontana P, De Filippo C, Cavalieri D, Donati C (2015) MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci Rep 5:1–7. https://doi.org/10.1038/srep09743
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Mirarab S, Nguyen N, Guo S, Wang L-S, Kim J, Warnow T (2015) PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J Comput Biol 22:377–386. https://doi.org/10.1089/cmb.2014.0156
Price MN, Dehal PS, Arkin AP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:1–10. https://doi.org/10.1371/journal.pone.0009490
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:1–11. https://doi.org/10.1371/journal.pone.0061217
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:1–18. https://doi.org/10.1186/gb-2011-12-6-r60
Cole AJ, Tulsankar SS, Saunders BJ, Fotedar R (2019) Effects of pond age and a commercial substrate (the water cleanser™) on natural productivity, bacterial abundance, nutrient concentrations, and growth and survival of marron (Cherax cainii Austin, 2002) in semi-intensive pond culture. Aquaculture 502:242–249. https://doi.org/10.1016/j.aquaculture.2018.12.046
Downs TM, Schallenberg M, Burns CW (2008) Responses of lake phytoplankton to micronutrient enrichment: a study in two New Zealand lakes and an analysis of published data. Aquat Sci 70:347–360. https://doi.org/10.1007/s00027-008-8065-6
Thakur DP, Lin CK (2003) Water quality and nutrient budget in closed shrimp (Penaeus monodon) culture systems. Aquac Eng 27:159–176. https://doi.org/10.1016/S0144-8609(02)00055-9
Shrestha MK, Lin CK (1996) Phosphorus fertilization strategy in fish ponds based on sediment phosphorus saturation level. Aquaculture 142:207–219. https://doi.org/10.1016/0044-8486(95)01214-1
Diana JS, Dettweiler DJ, Lin CK (1991) Effect of Nile tilapia (Oreochromis niloticus) on the ecosystem of aquaculture ponds, and its significance to the trophic cascade hypothesis. Can J Fish Aquat Sci 48:183–190. https://doi.org/10.1139/f91-025
Padrão J, Mota DK, Nicolau A, Mota M (2016) Growth optimization of marine diatom Amphora sp. by tailoring silica and nitrate concentration. Front Mar Sci 5:1–12. https://doi.org/10.3389/conf.FMARS.2018.06.00100
Raven JA (2016) Chloride: essential micronutrient and multifunctional beneficial ion. J Exp Bot 68:359–367. https://doi.org/10.1093/jxb/erw421
Civitello DJ, Hite JL, Hall SR (2014) Potassium enrichment stimulates the growth and reproduction of a clone of Daphnia dentifera. Oecologia 175:773–780. https://doi.org/10.1007/s00442-014-2943-5
Nordgreen A, Penglase S, Hamre K (2013) Increasing the levels of the essential trace elements Se, Zn, Cu and Mn in rotifers (Brachionus plicatilis) used as live feed. Aquaculture 380:120–129. https://doi.org/10.1016/j.aquaculture.2012.11.032
Gamboa-Delgado J (2014) Nutritional role of natural productivity and formulated feed in semi-intensive shrimp farming as indicated by natural stable isotopes. Rev Aquac 6:36–47. https://doi.org/10.1111/raq.12023
Jones CM (1995) Production of juvenile redclaw crayfish, Cherax quadricarinatus (von Martens)(Decapoda, Parastacidae) III. Managed pond production trials. Aquaculture 138:247–255. https://doi.org/10.1016/0044-8486(95)00067-4
Brown PB, Wetzel JE, Spacie A, Konopka A (1992) Evaluation of naturally-occurring organisms as food for juvenile crayfish Procambarus clarkii. J World Aquacult Soc 23:211–216
Jussila J, Mannonen A (1997) Marron (Cherax tenuimanus) and noble crayfish (Astacus astacus) hepatopancreas energy and its relationship to moisture content. Aquaculture 149:157–161. https://doi.org/10.1016/S0044-8486(96)01425-1
Jussila J (1999) Comparison of selected condition indices between intermolt and post-molt marron, Cherax tenuimanus, of different feeding status raised under intensive culture conditions. J Appl Aquac 9:57–66. https://doi.org/10.1300/J028v09n03_05
Wilder MN, Jasmani S, Jayasankar V, Kaneko T, Aida K, Hatta T, Nemoto S, Wigginton A (2009) Hemolymph osmolality, ion concentrations and calcium in the structural organization of the cuticle of the giant freshwater prawn Macrobrachium rosenbergii: changes with the molt cycle. Aquaculture 292:104–110. https://doi.org/10.1016/j.aquaculture.2009.03.034
Depledge M, Bjerregaard P (1989) Haemolymph protein composition and copper levels in decapod crustaceans. Helgoländer Meeresun 43:207–223
Alcorlo P, Otero M, Crehuet M, Baltanás A, Montes C (2006) The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci Total Environ 366:380–390. https://doi.org/10.1016/j.scitotenv.2006.02.023
Taylor H, Anstiss JM (1999) Copper and haemocyanin dynamics in aquatic invertebrates. Mar Freshw Res 50:907–931. https://doi.org/10.1071/MF99117
Foysal MJ, Fotedar R, Siddik MA, Tay A (2020) Lactobacillus acidophilus and L. plantarum improve health status, modulate gut microbiota and innate immune response of marron (Cherax cainii). Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-62655-y
Parrillo L, Coccia E, Volpe MG, Siano F, Pagliarulo C, Scioscia E, Varricchio E, Safari O, Eroldogan T, Paolucci M (2017) Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 473:161–168. https://doi.org/10.1016/j.aquaculture.2017.02.013
Foysal MJ, Fotedar R, Tay C-Y, Gupta SK (2019) Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 7:1–24. https://doi.org/10.7717/peerj.6891
Qian Y, Shi J, Chen Y, Lou L, Cui X, Cao R, Li P, Tang J (2010) Characterization of phosphate solubilizing bacteria in sediments from a shallow eutrophic lake and a wetland: isolation, molecular identification and phosphorus release ability determination. Molecules 15:8518–8533. https://doi.org/10.3390/molecules15118518
Wan W, Qin Y, Wu H, Zuo W, He H, Tan J, Wang Y, He D (2020) Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front Microbiol 11:1–15. https://doi.org/10.3389/fmicb.2020.00752
Liu S, Qi C, Jia Y, Gu Z, Li E (2020) Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 524:1–12. https://doi.org/10.1016/j.aquaculture.2020.735256
Bean N, Maloney E, Potter M, Korazemo P, Ray B, Taylor J, Seigler S, Snowden J (1998) Crayfish: a newly recognized vehicle for Vibrio infections. Epidemiol Infect 121:269–273. https://doi.org/10.1017/S0950268898001381
Ambas I, Suriawan A, Fotedar R (2013) Immunological responses of customised probiotics-fed marron, Cherax tenuimanus, (Smith 1912) when challenged with Vibrio mimicus. Fish Shellfish Immunol 35:262–270. https://doi.org/10.1016/j.fsi.2013.04.026
Acknowledgements
The authors are thankful to Mr. and Mrs. Hall for allowing the collection of plankton and marron from their farm.
Author information
Authors and Affiliations
Contributions
Smita Sadanand Tulsankar: conceptualization, designing and set up of the experiment, day to day feeding, data collection, data analysis and writing of the manuscript. Md. Javed Foysal: marron gut microbiota analysis, writing and reviewing the manuscript. Anthony J. Cole: plankton analysis, writing, reviewing and editing the manuscript. Monique Marthe Gagnon: designing experiment, supervision, reviewing and editing the manuscript. Ravi K. Fotedar: conceptualization, supervision, methodology validation, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Animal ethics approval is not mandatory for the invertebrate animal studies at Curtin University, Australia. However, all the required protocols were followed while handling the animals, as per the guidelines of Animal Welfare Act, Western Australia and the Australian Code for the Care and Use of Animals for Scientific Purposes (NHMRC, 2013).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Submission Declaration
The manuscript has not been published previously and accepted for publication elsewhere or it is not under consideration for publication elsewhere. The submitted manuscript has been approved by all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Tulsankar, S.S., Foysal, M.J., Cole, A.J. et al. A Mixture of Manganese, Silica and Phosphorus Supplementation Alters the Plankton Density, Species Diversity, Gut Microbiota and Improved the Health Status of Cultured Marron (Cherax cainii, Austin and Ryan, 2002). Biol Trace Elem Res 200, 1383–1394 (2022). https://doi.org/10.1007/s12011-021-02721-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02721-2