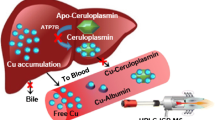
Abstract
The objective of the present study was assessment of the major copper and zinc species in dairy cow blood serum using a hybrid high-performance liquid chromatography-inductively coupled plasma-mass spectrometry (HPLC-ICP-MS) technique. A total of seventeen 5–6-year-old female Simmental cows, cultivated in the Southern Ural region, were examined. Speciation of serum Cu and Zn was performed using chromatographic PerkinElmer Series 200 system equipped with Agilent Bio SEC-5 Column and docked with NexION 300D mass spectrometer. Analysis of serum 63Cu species revealed four major fractions containing 2.5% (A), 15.6% (B), 75.6% (C), and 11.9% (D) of total copper levels. The revealed fractions could be assigned to tetrameric and dimeric macroglobulin, ceruloplasmin, albumin, and low molecular mass (LMM) copper compounds, respectively. Minor fraction (E) containing <1% of total serum Cu levels may be represented by low-molecular mass Cu species. Speciation analysis also revealed four Zn fractions containing 6.3% (A), 16.9% (B), 71% (C), and 3% (D) of total Zn levels that may be attributed to zinc-bound tetrameric and dimeric macroglobulin, albumin, and Zn-amino acid compounds. Correlation analysis demonstrated that relative levels (%) of Zn-B (dimeric α2-macroglobulin), Zn-C (albumin), and Zn-D (LMM) fractions correlate inversely with Cu-A (monomeric α2-macroglobulin) (r = −0.600), Cu-D (albumin) (r = −0.696), and Cu-C (ceruloplasmin) (r = −0.652), respectively. The obtained data demonstrate the particular features of Zn and Cu transport in dairy cows that may be used for assessment of dietary status of trace elements.



Similar content being viewed by others
References
Hill GM, Shannon MC (2019) Copper and zinc nutritional issues for agricultural animal production. Biol Trace Elem Res 188:148–159. https://doi.org/10.1007/s12011-018-1578-5
Ianni A, Innosa D, Martino C, Grotta L, Bennato F, Martino G (2019) Zinc supplementation of Friesian cows: effect on chemical-nutritional composition and aromatic profile of dairy products. J Dairy Sci 102:2918–2927. https://doi.org/10.3168/jds.2018-15868
Wysocka D, Snarska A, Sobiech P (2019) Copper - an essential micronutrient for calves and adult cattle. J Elem 24:101–110. https://doi.org/10.5601/jelem.2018.23.2.1645
Brugger D, Windisch WM (2017) Strategies and challenges to increase the precision in feeding zinc to monogastric livestock. Anim Nutr 3:103–108. https://doi.org/10.1016/j.aninu.2017.03.002
Osredkar J, Sustar N (2011) Copper and zinc, biological role and significance of copper/zinc imbalance. J Clin Toxicol 3:0495. https://doi.org/10.4172/2161-0495.S3-001
Smith SL, Grace ND, West DM, Balemi SC (2010) The impact of high zinc intake on the copper status of dairy cows in New Zealand. N Z V J 58:142–145. https://doi.org/10.1080/00480169.2010.67516
Twomey PJ, Viljoen A, Reynolds TM, Wierzbicki AS (2008) Non-ceruloplasmin-bound copper in routine clinical practice in different laboratories. J Trace Elem Med Biol 22:50–53. https://doi.org/10.1016/j.jtemb.2007.11.001
Hussein HA, Staufenbiel R (2012) Variations in copper concentration and ceruloplasmin activity of dairy cows in relation to lactation stages with regard to ceruloplasmin to copper ratios. Biol Trace Elem Res 146:47–52. https://doi.org/10.1007/s12011-011-9226-3
Iskandar M, Swist E, Trick KD, Wang B, L'Abbé MR, Bertinato J (2005) Copper chaperone for Cu/Zn superoxide dismutase is a sensitive biomarker of mild copper deficiency induced by moderately high intakes of zinc. Nutr J 4:35. https://doi.org/10.1186/1475-2891-4-35
Suttle NF (2012) Copper imbalances in ruminants and humans: unexpected common ground. Adv Nutr 3:666–674. https://doi.org/10.3945/an.112.002220
Michalke B (2003) Element speciation definitions, analytical methodology, and some examples. Ecotoxicol Environ Saf 56:122–139. https://doi.org/10.1016/S0147-6513(03)00056-3
Cabrera A, Alonzo E, Sauble E, Chu Y-L, Linder MC, Sato DS, Mason AZ (2008) Copper binding components of plasma and cytoplasm, copper turnover and excretion, as determined in the mouse with large doses of 65Cu. Biometals 21:525–543. https://doi.org/10.1007/s10534-008-9139-6
Mir SH, Mani V, Pal RP, Malik TA, Sharma H (2020) Zinc in ruminants: metabolism and homeostasis. Proc Natl Acad Sci, India Sect B: Biol Sci 90:9–19. https://doi.org/10.1007/s40011-018-1048-z
Martino FAR, Sánchez MLF, Medel AS (2002) Multi-elemental fractionation in milk whey by size exclusion chromatography coupled on line to ICP-MS. J Anal At Spectrom 17:1271–1277. https://doi.org/10.1039/B201890J
Miroshnikov SA, Zavyalov OA, Frolov AN et al (2017) The reference intervals of hair trace element content in hereford cows and heifers (Bos taurus). Biol Trace Elem Res 180:56–62. https://doi.org/10.1007/s12011-017-0991-5
National Research council (2005) Mineral tolerance of animals. National Academy Press
USSR State Agriculture Committee (1987) Temporary maximum allowable levels of certain chemical elements and gossypol in feeds for farm animals and feed additives. Gosagroprom USSR, Moscow
National Research Council (2001) Nutrient requirements of dairy cattle. National Academy Press, Washington, DC
Balkhi SE, Poupon J, Trocello JM, Massicot F, Woimant F, Laprevote O (2010) Human plasma copper proteins speciation by size exclusion chromatography coupled to inductively coupled plasma mass spectrometry. Solutions for columns calibration by sulfur detection. Anal Chem 82:6904–6910. https://doi.org/10.1021/ac101128x
Nischwitz V, Berthele A, Michalke B (2008) Speciation analysis of selected metals and determination of their total contents in paired serum and cerebrospinal fluid samples: an approach to investigate the permeability of the human blood-cerebrospinal fluid-barrier. Anal Chim Acta 627:258–269. https://doi.org/10.1016/j.aca.2008.08.018
Bewley TA (1977) Optical activity of disulfide bonds in proteins. 1. Studies on plasmin modified human somatotropin. Biochemistry 16:209–215. https://doi.org/10.1021/bi00621a008
Willkommen D, Lucio M, Schmitt-Kopplin P, Gazzaz M, Schroeter M, Sigaroudi A, Michalke B (2018) Species fractionation in a case-control study concerning Parkinson’s disease: Cu-amino acids discriminate CSF of PD from controls. J Trace Elem Med Biol 49:164–170. https://doi.org/10.1016/j.jtemb.2018.01.005
Yokus B, Cakir UD (2006) Seasonal and physiological variations in serum chemistry and mineral concentrations in cattle. Biol Trace Elem Res 109:255–266. https://doi.org/10.1385/BTER:109:3:255
Spolders M, Höltershinken M, Meyer U, Rehage J, Flachowsky G (2010) Assessment of reference values for copper and zinc in blood serum of first and second lactating dairy cows. Vet Med Int Article ID 194656. https://doi.org/10.4061/2010/194656
Noaman V, Rasti M, Ranjbari AR, Shirvani E (2012) Serum copper, zinc and iron status of various bovine categories on Holstein dairy cattle farms. Comp Clin Pathol 21:1727–1731. https://doi.org/10.1007/s00580-011-1357-6
Linder MC, Lomeli NA, Donley S, Mehrbod F, Cerveza P, Cotton S, Wooten L (1999) Copper transport in mammals. In: Leone A, Mercer JFB (eds) Copper transport and its disorders, vol 448. Springer, Boston, pp 1–16. https://doi.org/10.1007/978-1-4615-4859-1_1
Linder MC (2016) Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics 8:887–905. https://doi.org/10.1039/C6MT00103C
Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N (1998) Copper transport. Am J Clin Nutr 67:965S–971S. https://doi.org/10.1093/ajcn/67.5.965S
Inagaki K, Mikuriya N, Morita S, Haraguchi H, Nakahara Y, Hattori M, Kinosita T, Saito H (2000) Speciation of protein-binding zinc and copper in human blood serum by chelating resin pre-treatment and inductively coupled plasma mass spectrometry. Analyst 125:197–203. https://doi.org/10.1039/A907088E
Ledesma-Osuna AI, Ramos-Clamont G, Vázquez-Moreno L (2008) Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim Pol 55:491–497. https://doi.org/10.18388/abp.2008_3054
Twomey PJ, Viljoen A, House IM, Reynolds TM, Wierzbicki AS (2005) Relationship between serum copper, ceruloplasmin, and non–ceruloplasmin-bound copper in routine clinical practice. Clin Chem 51:1558–1559. https://doi.org/10.1373/clinchem.2005.052688
Collins KL, Roberts EA, Adeli K, Bohn D, Harvey EA (2008) Single pass albumin dialysis (SPAD) in fulminant Wilsonian liver failure: a case report. Pediatr Nephrol 23:1013–1016. https://doi.org/10.1007/s00467-008-0761-x
Choi BS, Zheng W (2009) Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res 1248:14–21. https://doi.org/10.1016/j.brainres.2008.10.056
Løvstad RA (2004) A kinetic study on the distribution of Cu (II)-ions between albumin and transferrin. BioMetals 17:111–113. https://doi.org/10.1023/B:BIOM.0000018362.37471.0b
Brumas V, Alliey N, Berthon G (1993) A new investigation of copper (II)-serine, copper (II)-histidine-serine, copper (II)-asparagine, and copper (II)-histidine-asparagine equilibria under physiological conditions, and implications for simulation models relative to blood plasma. J Inorg Biochem 52:287–296. https://doi.org/10.1016/0162-0134(93)80032-5
Kahn N, Van Loon JC (1979) Atomic absorption spectrophotometry as a chromatography detector for copper-amino acid complexes in human serum. J Liq Chromatogr 2:23–36. https://doi.org/10.1080/01483917908060042
Masuoka J, Saltman P (1994) Zinc (II) and copper (II) binding to serum albumin. A comparative study of dog, bovine, and human albumin. J Biol Chem 269:25557–25561
Bal W, Sokołowska M, Kurowska E, Faller P (2013) Binding of transition metal ions to albumin: sites, affinities and rates. Biochim Biophys Acta, Gen Subj 1830:5444–5455. https://doi.org/10.1016/j.bbagen.2013.06.018
Adham NF, Song MK, Rinderknecht H (1977) Binding of zinc to alpha-2-macroglobulin and its role in enzyme binding activity. Biochim Biophys Acta Protein Struct 495:212–219. https://doi.org/10.1016/0005-2795(77)90378-6
Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Malavolta M (2006) Zinc-binding proteins (metallothionein and α-2 macroglobulin) and immunosenescence. Exp Gerontol 41:1094–1107. https://doi.org/10.1016/j.exger.2006.08.010
Liu N, Lo LSL, Askary SH, Jones L, Kidane TZ, Trang T, Nguyen M, Goforth J, Chu YH, Vivas E, Tsai M, Westbrook T, Linder MC (2007) Transcuprein is a macroglobulin regulated by copper and iron availability. J Nutr Biochem 18:597–608. https://doi.org/10.1016/j.jnutbio.2006.11.005
Thieme R, Kurz S, Kolb M, Debebe T, Holtze S, Morhart M, Huse K, Szafranski K, Platzer M, Hildebrandt TB, Birkenmeier G (2015) Analysis of alpha-2 macroglobulin from the long-lived and cancer-resistant naked mole-rat and human plasma. PLoS One 10:e0130470. https://doi.org/10.1371/journal.pone.0130470
Pace NJ, Weerapana E (2014) Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules 4:419–434. https://doi.org/10.3390/biom4020419
Ranjbar M, Shahsavan N, Yousefi M (2012) Synthesis and characterization of nano structured zinc (II) cysteine complex under ultrasound irradiation. Chem Sci Int J 2:111–121. https://doi.org/10.9734/ACSJ/2012/1617
Black JR, Kavner A, Schauble EA (2011) Calculation of equilibrium stable isotope partition function ratios for aqueous zinc complexes and metallic zinc. Geochim Cosmochim Acta 75:769–783. https://doi.org/10.1016/j.gca.2010.11.019
Kho R, Nguyen L, Torres-Martínez CL, Mehra RK (2000) Zinc–histidine as nucleation centers for growth of ZnS nanocrystals. Biochem Biophys Res Commun 272:29–35. https://doi.org/10.1006/bbrc.2000.2712
Mineo P, Vitalini D, La Mendola DIEGO, Rizzarelli E, Scamporrino E, Vecchio G (2002) Electrospray mass spectrometric studies of L-carnosine (β-alanyl-L-histidine) complexes with copper (II) or zinc ions in aqueous solution. Rapid Commun Mass Spectrom 16:722–729. https://doi.org/10.1002/rcm.633
Foley S, Enescu M (2007) A Raman spectroscopy and theoretical study of zinc–cysteine complexation. Vib Spectrosc 44:256–265. https://doi.org/10.1016/j.vibspec.2006.12.004
Altun Y, Köseoĝlu F (2005) Stability of copper (II), nickel (II) and zinc (II) binary and ternary complexes of histidine, histamine and glycine in aqueous solution. J Solut Chem 34:213–231. https://doi.org/10.1007/s10953-005-2763-7
Irving H, Williams RJP (1953) The stability of transition-metal complexes. J Chem Soc 637:3192–3210. https://doi.org/10.1039/JR9530003192
Szilágyi I, Labádi I, Hernadi K, Pálinkó I, Nagy NV, Korecz L, Rockenbauer A, Kele Z, Kiss T (2005) Speciation study of an imidazolate-bridged copper (II)–zinc (II) complex in aqueous solution. J Inorg Biochem 99:1619–1629. https://doi.org/10.1016/j.jinorgbio.2005.05.001
López-Alonso M, Miranda M (2020) Copper supplementation, a challenge in cattle. Animals 10:1890. https://doi.org/10.3390/ani10101890
Gaetke LM, Chow-Johnson HS, Chow CK (2014) Copper: toxicological relevance and mechanisms. Arch Toxicol 88:1929–1938. https://doi.org/10.1007/s00204-014-1355-y
López-Alonso M, Crespo A, Miranda M, Castillo C, Hernández J, Benedito JL (2006) Assessment of some blood parameters as potential markers of hepatic copper accumulation in cattle. J Vet Diagn Investig 18:71–75. https://doi.org/10.1177/104063870601800109
Acknowledgements
The study was performed in agreement with the plan of the studies of FSBSI FSC BST RAS № FNWZ-2019-0001.
Funding
The study was performed with the financial support of the Russian Science Foundation (project No. 078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. S1.
SEC-ICP-MS chromatograms of the copper blood serum species of the same sample obtained during the initial and repeated sampling (PNG 194 kb)
Rights and permissions
About this article
Cite this article
Miroshnikov, S.A., Notova, S.V., Skalnaya, M.G. et al. Speciation of Serum Copper and Zinc-Binding High- and Low-Molecular Mass Ligands in Dairy Cows Using HPLC-ICP-MS Technique. Biol Trace Elem Res 200, 591–599 (2022). https://doi.org/10.1007/s12011-021-02666-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02666-6