Abstract
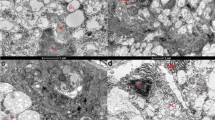
Nickel is an industrial and environmental toxic metal, which is toxic to humans in certain forms at high doses. Here, we investigated the cytotoxic effects of nickel sulfate (NiSO4) exposure on the human thyroid follicular epithelial cells (Nthy-ori 3-1) and its underlying toxicological mechanisms. The results showed that NiSO4 reduced the cell viability of Nthy-ori 3-1 cells in a dose- and time-dependent manner, inducing S and G2/M phases cell-cycle arrest and apoptosis. Electron microscopy demonstrated that abundant autophagic vacuoles were found in Nthy-ori 3-1 cells after NiSO4 treatment. Accordingly, exposure of Nthy-ori 3-1 cells to NiSO4 resulted in a dose-dependent increase of LC3II/I ratio, an induction of Beclin-1 expression, and a decrease in p62 levels. Blockade of autophagy with 3-methyladenine (3-MA) potentiated the NiSO4-induced apoptotic cell death, while induction of autophagy significantly alleviated toxicity of NiSO4. From a molecular standpoint, NiSO4 markedly promoted the activation of p38 and IKKβ by increasing their phosphorylation. In conclusion, we showed that autophagy was induced to protect thyroid cells from Ni2+ mediated apoptosis, thus providing rational strategy to prevent against nickel toxicity in the thyroid.






Similar content being viewed by others
References
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17(3). https://doi.org/10.3390/ijerph17030679
Vasiluk L, Sowa J, Sanborn P, Ford F, Dutton MD et al (2019) Bioaccessibility estimates by gastric SBRC method to determine relationships to bioavailability of nickel in ultramafic soils. Sci Total Environ 673:685–693. https://doi.org/10.1016/j.scitotenv.2019.04.059
Pappas RS, Fresquez MR, Martone N, Watson CH (2014) Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J Anal Toxicol 38(4):204–211. https://doi.org/10.1093/jat/bku013
Son YO (2019) Molecular mechanisms of nickel-induced carcinogenesis. Endocr Metab Immune Disord Drug Targets. https://doi.org/10.2174/1871530319666191125112728
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A et al (2015) Executive summary to EDC-2: the Endocrine Society's Second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev 36(6):593–602. https://doi.org/10.1210/er.2015-1093
Kabir ER, Rahman MS, Rahman I (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40(1):241–258. https://doi.org/10.1016/j.etap.2015.06.009
Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G et al (2017) Thyroid hormones and cardiovascular disease. Nat Rev Cardiol 14(1):39–55. https://doi.org/10.1038/nrcardio.2016.174
Wirth EK, Meyer F (2017) Neuronal effects of thyroid hormone metabolites. Mol Cell Endocrinol 458:136–142. https://doi.org/10.1016/j.mce.2017.01.007
Chung SM, Moon JS, Yoon JS, Won KC, Lee HW (2019) Sex-specific effects of blood cadmium on thyroid hormones and thyroid function status: Korean nationwide cross-sectional study. J Trace Elem Med Biol 53:55–61. https://doi.org/10.1016/j.jtemb.2019.02.003
Dolgova NV, Nehzati S, MacDonald TC, Summers KL, Crawford AM et al (2019) Disruption of selenium transport and function is a major contributor to mercury toxicity in zebrafish larvae. Metallomics 11(3):621–631. https://doi.org/10.1039/c8mt00315g
Zadjali SA, Nemmar A, Fahim MA, Azimullah S, Subramanian D et al (2015) Lead exposure causes thyroid abnormalities in diabetic rats. Int J Clin Exp Med 8(5):7160–7167
Jain RB, Choi YS (2016) Interacting effects of selected trace and toxic metals on thyroid function. Int J Environ Health Res 26(1):75–91. https://doi.org/10.1080/09603123.2015.1020416
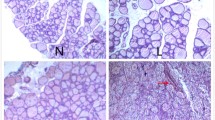
Liu Y, Chen H, Zhang L, Zhang T, Ren X (2020) The association between thyroid injury and apoptosis, and alterations of Bax, Bcl-2, and caspase-3 mRNA/protein expression induced by nickel sulfate in Wistar rats. Biol Trace Elem Res 195(1):159–168. https://doi.org/10.1007/s12011-019-01825-0
Yao Y, Costa M (2014) Toxicogenomic effect of nickel and beyond. Arch Toxicol 88(9):1645–1650. https://doi.org/10.1007/s00204-014-1313-8
Guo H, Chen L, Cui H, Peng X, Fang J et al (2015) Research advances on pathways of nickel-induced apoptosis. Int J Mol Sci 17(1). https://doi.org/10.3390/ijms17010010
Zheng GH, Liu CM, Sun JM, Feng ZJ, Cheng C (2014) Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol 147:105–111. https://doi.org/10.1016/j.aquatox.2013.12.015
Towers CG, Thorburn A (2016) Therapeutic targeting of autophagy. EBioMedicine 14:15–23. https://doi.org/10.1016/j.ebiom.2016.10.034
Yoshii SR, Mizushima N (2017) Monitoring and measuring autophagy. Int J Mol Sci 18(9). https://doi.org/10.3390/ijms18091865
Zheng T, Xu C, Mao C, Mou X, Wu F et al (2018) Increased interleukin-23 in Hashimoto's thyroiditis disease induces autophagy suppression and reactive oxygen species accumulation. Front Immunol 9:96. https://doi.org/10.3389/fimmu.2018.00096
Xu C, Wu F, Mao C, Wang X, Zheng T et al (2016) Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J Autoimmun 75:50–57. https://doi.org/10.1016/j.jaut.2016.07.008
Kurashige T, Nakajima Y, Shimamura M, Matsuyama M, Yamada M et al (2019) Basal autophagy deficiency causes thyroid follicular epithelial cell death in mice. Endocrinology 160(9):2085–2092. https://doi.org/10.1210/en.2019-00312
Son YO, Pratheeshkumar P, Divya SP, Zhang Z, Shi X (2017) Nuclear factor erythroid 2-related factor 2 enhances carcinogenesis by suppressing apoptosis and promoting autophagy in nickel-transformed cells. J Biol Chem 292(20):8315–8330. https://doi.org/10.1074/jbc.M116.773986
Kang YT, Hsu WC, Wu CH, Hsin IL, Wu PR et al (2017) Metformin alleviates nickel-induced autophagy and apoptosis via inhibition of hexokinase-2, activating lipocalin-2, in human bronchial epithelial cells. Oncotarget 8(62):105536–105552. https://doi.org/10.18632/oncotarget.22317
Lemoine NR, Mayall ES, Jones T, Sheer D, McDermid S et al (1989) Characterisation of human thyroid epithelial cells immortalised in vitro by simian virus 40 DNA transfection. Br J Cancer 60(6):897–903. https://doi.org/10.1038/bjc.1989.387
Gong A, Ye S, Xiong E, Guo W, Zhang Y et al (2013) Autophagy contributes to ING4-induced glioma cell death. Exp Cell Res 319(12):1714–1723. https://doi.org/10.1016/j.yexcr.2013.05.004
Pan YL, Xin R, Wang SY, Wang Y, Zhang L et al (2018) Nickel-smelting fumes induce mitochondrial damage and apoptosis, accompanied by decreases in viability, in NIH/3T3 cells. Arch Biochem Biophys 660:20–28. https://doi.org/10.1016/j.abb.2018.10.008
Siddiqui MA, Ahamed M, Ahmad J, Majeed Khan MA, Musarrat J et al (2012) Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that is abrogated by the dietary antioxidant curcumin. Food Chem Toxicol 50(3-4):641–647. https://doi.org/10.1016/j.fct.2012.01.017
Wu Y, Kong L (2020) Advance on toxicity of metal nickel nanoparticles. Environ Geochem Health 42(7):2277–2286. https://doi.org/10.1007/s10653-019-00491-4
Cambre MH, Holl NJ, Wang B, Harper L, Lee HJ et al (2020) Cytotoxicity of NiO and Ni(OH)(2) nanoparticles is mediated by oxidative stress-induced cell death and suppression of cell proliferation. Int J Mol Sci 21(7). https://doi.org/10.3390/ijms21072355
Terpilowska S, Siwicki AK (2019) Cell cycle and transmembrane mitochondrial potential analysis after treatment with chromium(iii), iron(iii), molybdenum(iii) or nickel(ii) and their mixtures. Toxicol Res (Camb) 8(2):188–195. https://doi.org/10.1039/c8tx00233a
Ma C, Song M, Zhang Y, Yan M, Zhang M et al (2014) Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicol Rep 1:114–121. https://doi.org/10.1016/j.toxrep.2014.04.008
Wu Y, Ma J, Sun Y, Tang M, Kong L (2020) Effect and mechanism of PI3K/AKT/mTOR signaling pathway in the apoptosis of GC-1 cells induced by nickel nanoparticles. Chemosphere 255:126913. https://doi.org/10.1016/j.chemosphere.2020.126913
Cavalli G, Cenci S (2020) Autophagy and protein secretion. J Mol Biol 432(8):2525–2545. https://doi.org/10.1016/j.jmb.2020.01.015
Li X, He S, Ma B (2020) Autophagy and autophagy-related proteins in cancer. Mol Cancer 19(1):12. https://doi.org/10.1186/s12943-020-1138-4
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A et al (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8(4):445–544. https://doi.org/10.4161/auto.19496
Das S, Reddy RC, Chadchan KS, Patil AJ, Biradar MS et al (2020) Nickel and oxidative stress: cell signaling mechanisms and protective role of vitamin C. Endocr Metab Immune Disord Drug Targets 20(7):1024–1031. https://doi.org/10.2174/1871530319666191205122249
Lee P, Chandel NS, Simon MC (2020) Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol 21(5):268–283. https://doi.org/10.1038/s41580-020-0227-y
Martínez-Limón A, Joaquin M, Caballero M, Posas F, de Nadal E (2020) The p38 pathway: from biology to cancer therapy. Int J Mol Sci 21(6). https://doi.org/10.3390/ijms21061913
Athamneh K, Alneyadi A, Alsamri H, Alrashedi A, Palakott A et al (2020) Origanum majorana essential oil triggers p38 MAPK-mediated protective autophagy, apoptosis, and caspase-dependent cleavage of P70S6K in colorectal cancer cells. Biomolecules 10(3). https://doi.org/10.3390/biom10030412
Comb WC, Cogswell P, Sitcheran R, Baldwin AS (2011) IKK-dependent, NF-κB-independent control of autophagic gene expression. Oncogene 30(14):1727–1732. https://doi.org/10.1038/onc.2010.553
Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E et al (2010) The IKK complex contributes to the induction of autophagy. EMBO J 29(3):619–631. https://doi.org/10.1038/emboj.2009.364
Zhong W, Zhu H, Sheng F, Tian Y, Zhou J et al (2014) Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells. Autophagy 10(7):1285–1300. https://doi.org/10.4161/auto.28789
Thévenod F, Lee WK (2013) Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol 87(10):1743–1786. https://doi.org/10.1007/s00204-013-1110-9
Han J, Wu J, Silke J (2020) An overview of mammalian p38 mitogen-activated protein kinases, central regulators of cell stress and receptor signaling. F1000Res 9. https://doi.org/10.12688/f1000research.22092.1
Liu K, Zhang L, Zhao Q, Zhao Z, Zhi F et al (2018) SKP2 attenuates NF-κB signaling by mediating IKKβ degradation through autophagy. J Mol Cell Biol 10(3):205–215. https://doi.org/10.1093/jmcb/mjy012
Acknowledgements
The authors thank all those who participated in this study.
Funding
This study was supported by the Program for National Natural Science Foundation of China (31670518).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, T., Chen, H. & Liu, Y. Nickel Sulfate Induces Autophagy in Human Thyroid Follicular Epithelial Cells. Biol Trace Elem Res 200, 122–133 (2022). https://doi.org/10.1007/s12011-021-02643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02643-z