Abstract
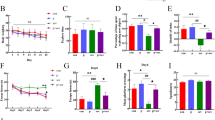
Lanthanum can induce neurotoxicity and impair cognitive function; therefore, research on the mechanism by which the ability to learning and memory is decreased by lanthanum is vitally important for protecting health. Microglia are a type of neuroglia located throughout the brain and spinal cord that play an important role in the central nervous system. When overactive, these cells can cause the excessive production of inflammatory cytokines that can damage neighboring neurons. The purpose of this study was to explore the effect of lanthanum in the form of lanthanum chloride (LaCl3) on learning and the memory of mice and determine whether there is a relationship between hippocampal neurons or learning and memory damage and excessive production of inflammatory cytokines. Four groups of pregnant Chinese Kun Ming mice were exposed to 0, 18, 36, or 72 mM LaCl3 in their drinking water during lactation. The offspring were then exposed to LaCl3 in the breast milk at birth until weaning and then exposed to these concentrations in their drinking water for 2 months after weaning. The results showed that LaCl3 impaired learning and memory in mice and injured their neurons, activated the microglia, and significantly overregulated the mRNA and protein expression of tumor necrosis factor alpha, interleukin (IL)-1β, IL-6, monocyte chemoattractant protein-1, and nitric oxide in the hippocampus. The results of this study suggest that lanthanum can impair learning and memory in mice, possibly by over-activating the microglia.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code Availability
Not applicable.
References
Yang J, Liu Q, Zhang L, Wu S, Qi M, Lu S, Xi Q, Cai Y (2009) Lanthanum chloride impairs memory, decreases pCaMK IV, pMAPK and pCREB expression of hippocampus in rats. Toxicol Lett 190(2):208–214. https://doi.org/10.1016/j.toxlet.2009.07.016
Feng L, He X, Xiao H, Li Z, Li F, Liu N, Chai Z, Zhao Y, Zhang Z (2007) Ytterbium and trace element distribution in brain and organic tissues of offspring rats after prenatal and postnatal exposure to ytterbium. Biol Trace Elem Res 117(1-3):89–104. https://doi.org/10.1007/BF02698086
Fan G, Yuan Z, Zheng H, Liu Z (2004) Study on the effects of exposure to rare earth elements and health-responses in children aged 7-10 years. Journal of Hygiene Research 33(1):23–28 (in Chisese)
Xu H (2004) The progress of resource, environment and health in China. Peking University Medical Press, Beijing, pp. 43–64 (in Chisese)
Feng L, Xiao H, He X, Li Z, Li F, Liu N, Zhao Y, Huang Y, Zhang Z, Chai Z (2006) Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol Lett 165(2):112–120. https://doi.org/10.1016/j.toxlet.2006.02.003
Feng L, Xiao H, He X, Li Z, Li F, Liu N, Chai Z, Zhao Y, Zhang Z (2006) Long-term effects of lanthanum intake on the neurobehavioral development of the rat. Neurotoxicol Teratol 28(1):119–124. https://doi.org/10.1016/j.ntt.2005.10.007
Hu X, Yang J, Sun Y, Gao X, Zhang L, Li Y, Yu M, Liu S, Lu X, Jin C, Wu S, Cai Y (2018) Lanthanum chloride impairs memory in rats by disturbing the glutamate-glutamine cycle and over-activating NMDA receptors. Food Chem Toxicol 113:1–13. https://doi.org/10.1016/j.fct.2018.01.023
Valente LA, Begg LR, Filiano AJ (2019) Updating neuroimmune targets in central nervous system dysfunction. Trends Pharmacol Sci 40(7):482–494. https://doi.org/10.1016/j.tips.2019.04.013
Geirsdottir L, David E, Keren-Shaul H, Weiner A, Bohlen SC, Neuber J, Balic A, Giladi A, Sheban F, Dutertre CA, Pfeifle C, Peri F, Raffo-Romero A, Vizioli J, Matiasek K, Scheiwe C, Meckel S, Matz-Rensing K, van der Meer F, Thormodsson FR, Stadelmann C, Zilkha N, Kimchi T, Ginhoux F, Ulitsky I, Erny D, Amit I, Prinz M (2019) Cross-species single-cell analysis reveals divergence of the primate microglia program. Cell 179(7):1609–1622 e1616. https://doi.org/10.1016/j.cell.2019.11.010
Hou BR, Jiang C, Wang ZN, Ren HJ (2020) Exosome-mediated crosstalk between microglia and neural stem cells in the repair of brain injury. Neural Regen Res 15(6):1023–1024. https://doi.org/10.4103/1673-5374.270302
Yan L, Yang J, Yu M, Lu Y, Huang L, Wang J, Lu X, Jin C, Wu S, Cai Y (2019) Lanthanum chloride induces neuron damage by activating the nuclear factor-kappa B signaling pathway in activated microglia. Metallomics 11(7):1277–1287. https://doi.org/10.1039/c9mt00108e
Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK (2017) Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI insight 2(7):e91229. https://doi.org/10.1172/jci.insight.91229
Helmy A, Guilfoyle MR, Carpenter KLH, Pickard JD, Menon DK, Hutchinson PJ (2016) Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury. J Cereb Blood Flow Metab 36(8):1434–1448. https://doi.org/10.1177/0271678X15620204
Huang LQ, Zhu GF, Deng YY, Jiang WQ, Fang M, Chen CB, Cao W, Wen MY, Han YL, Zeng HK (2014) Hypertonic saline alleviates cerebral edema by inhibiting microglia-derived TNF-alpha and IL-1beta-induced Na-K-Cl Cotransporter up-regulation. J Neuroinflammation 11:102. https://doi.org/10.1186/1742-2094-11-102
Madhu K, Prakash T (2018) Asiaticoside counteracts the in vitro activation of microglia and astrocytes: innuendo for multiple sclerosis. Biomed Pharmacother 107:303–305. https://doi.org/10.1016/j.biopha.2018.08.010
Deng W, Mandeville E, Terasaki Y, Li W, Holder J, Chuang AT, Ning M, Arai K, Lo EH, Xing C (2020) Transcriptomic characterization of microglia activation in a rat model of ischemic stroke. J Cereb Blood Flow Metab 40(1_suppl):S34–S48. https://doi.org/10.1177/0271678X20932870
Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47. https://doi.org/10.1186/1750-1326-4-47
Blank T, Prinz M (2013) Microglia as modulators of cognition and neuropsychiatric disorders. Glia 61(1):62–70. https://doi.org/10.1002/glia.22372
D'Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36(1):60–90. https://doi.org/10.1016/s0165-0173(01)00067-4
Zhu W, Xu S, Shao P, Zhang H, Wu D, Yang W, Feng J (1997) Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol Trace Elem Res 57(1):71–77. https://doi.org/10.1007/BF02803871
Summers MJ, Crowe SF, Ng KT (1996) Administration of lanthanum chloride following a reminder induces a transient loss of memory retrieval in day-old chicks. Brain Res Cogn Brain Res 4(2):109–119
Che Y, Cui Y, Jiang X (2009) Effects of lanthanum chloride administration in prenatal stage on one-trial passive avoidance learning in chicks. Biol Trace Elem Res 127(1):37–44. https://doi.org/10.1007/s12011-008-8225-5
Yang J, Liu Q, Wu S, Xi Q, Cai Y (2012) Effects of lanthanum chloride on glutamate level, intracellular calcium concentration and caspases expression in the rat hippocampus. BioMetals 26(1):43–59. https://doi.org/10.1007/s10534-012-9593-z
Rezaie P, Male D (2002) Mesoglia & microglia--a historical review of the concept of mononuclear phagocytes within the central nervous system. J Hist Neurosci 11(4):325–374. https://doi.org/10.1076/jhin.11.4.325.8531
Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39(1):151–170
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Wang F, Cui N, Yang L, Shi L, Li Q, Zhang G, Wu J, Zheng J, Jiao B (2015) Resveratrol rescues the impairments of hippocampal neurons stimulated by microglial over-activation in vitro. Cell Mol Neurobiol 35(7):1003–1015. https://doi.org/10.1007/s10571-015-0195-5
Wang HL, Ma RH, Fang H, Xue ZG, Liao QW (2015) Impaired spatial learning memory after isoflurane anesthesia or appendectomy in aged mice is associated with microglia activation. J Cell Death 8:9–19. https://doi.org/10.4137/JCD.S30596
Fetler L, Amigorena S (2005) Neuroscience. Brain under surveillance: the microglia patrol. Science 309(5733):392–393. https://doi.org/10.1126/science.1114852
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318. https://doi.org/10.1126/science.1110647
Landreth GE (2009) Microglia in central nervous system diseases. J Neuroimmune Pharmacol 4(4):369–370. https://doi.org/10.1007/s11481-009-9173-3
Taetzsch T, Block ML (2013) Pesticides, microglial NOX2, and Parkinson’s disease. J Biochem Mol Toxicol 27(2):137–149. https://doi.org/10.1002/jbt.21464
Malm TM, Jay TR, Landreth GE (2015) The evolving biology of microglia in Alzheimer’s disease. Neurotherapeutics 12(1):81–93. https://doi.org/10.1007/s13311-014-0316-8
Verina T, Kiihl SF, Schneider JS, Guilarte TR (2011) Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology 32(2):215–226. https://doi.org/10.1016/j.neuro.2010.11.003
Kumawat KL, Kaushik DK, Goswami P, Basu A (2014) Acute exposure to lead acetate activates microglia and induces subsequent bystander neuronal death via caspase-3 activation. Neurotoxicology 41:143–153. https://doi.org/10.1016/j.neuro.2014.02.002
Hu Z, Yu F, Gong P, Qiu Y, Zhou W, Cui Y, Li J, Chen H (2014) Subneurotoxic copper(II)-induced NF-kappaB-dependent microglial activation is associated with mitochondrial ROS. Toxicol Appl Pharmacol 276(2):95–103. https://doi.org/10.1016/j.taap.2014.01.020
Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, Carta AR (2014) Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson's disease. Neurobiol Dis 71:280–291. https://doi.org/10.1016/j.nbd.2014.08.011
Lloyd RV, Hanna PM, Mason RP (1997) The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med 22(5):885–888
Kalehua AN, Nagel JE, Whelchel LM, Gides JJ, Pyle RS, Smith RJ, Kusiak JW, Taub DD (2004) Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp Cell Res 297(1):197–211. https://doi.org/10.1016/j.yexcr.2004.02.031
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81673220, 81273117, and 81773469).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The manuscript does not contain clinical studies or patient data. All experiments and surgical procedures complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication, amended in 1996). The study was approved by the Local Ethics Committee (Laboratory Animal Welfare and Ethics Committee of the China Medical University, Shenyang, China).
Consent for Publication
Not applicable.
Conflict of Interest
The authors have declared that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Yan, L., Yang, J., Yu, M. et al. Lanthanum Impairs Learning and Memory by Activating Microglia in the Hippocampus of Mice. Biol Trace Elem Res 200, 1640–1649 (2022). https://doi.org/10.1007/s12011-021-02637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02637-x