Abstract
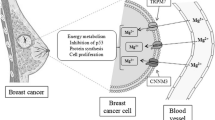
Reduced serum magnesium concentrations in women with breast cancer compromises one of the antioxidant defense system involved in the carcinogenesis process. To evaluate parameters of magnesium, the enzyme activity of superoxide dismutase, and its relation with oxidative stress markers in women with breast cancer. A case-control study was conducted, involving 60 women in the age range between 29 and 65 years, divided into two groups: women with breast cancer (n = 30) and women without breast cancer (n = 30). Plasma; ionized, erythrocytic, and urinary magnesium intake; plasma concentrations of thiobarbituric acid reactive substances; and erythrocyte superoxide dismutase enzyme activity were evaluated. The mean value of the amount of dietary magnesium was below the recommended level in both groups studied, with no statistical difference (p > 0.05). Plasma, ionized, and erythrocyte magnesium concentrations of women with breast cancer were reduced in relation to the control group (p < 0.0001) and inadequate according to the reference values. Urinary excretion was high, with a significant difference between groups (p < 0.0001). The mean concentration of thiobarbituric acid reactive substances was high in the study participants, with no significant statistical difference between the groups (p > 0.05). The mean values of superoxide dismutase enzyme activity were adequate, with no statistically significant difference between the groups (p > 0.05). Women with breast cancer have impaired magnesium homeostasis, characterized by its reduction in diet, plasma, and erythrocytes and its increase in urine.


Similar content being viewed by others
References
Baaij JHF, Hoenderop GJJ, Bindels RJM (2015) Magnesium in man: implications for health and disease. Physiol Rev 95:1–46
Blaszczyk U, Duda-Chodak A (2013) Magnesium: its role in nutrition and carcinogenesis. Roczniki Państwowego Zakładu Higieny 64(3):165–171
Volpe S (2013) Magnesium in disease prevention and overall health. Adv Nutr 4(3):378–383
Abdelgawad IA, El-Mously RH, Saber MM, Mansour OA, Shouman AS (2015) Significance of serum levels of vitamin D and some related minerals in breast cancer patients. Int J Clin Exp Pathol 8(4):4074–4082
Czerny B, Krupka K, Ożarowski M, Seremak-Mrozikiewicz A (2014) Screening of trace elements in hair of the female population with different types of cancers in Wielkopolska region of Poland. Sci World J 953181
Feng JF, Lu L, Zeng YH, Luo J, Yang YW, Wang D (2012) Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int J Clin Oncol 17(6):575–583
Karki K, Pande D, Negi R, Khanna S, Khanna RS, Khanna HD (2015b) Correlation of serum toll like receptor 9 and trace elements with lipid peroxidation in the patients of breast diseases. J Trace Elem Med Biol 30:11–16
Tao MH, Dai Q, Millen AE, Nie J, Edge SB, Trevisan M, Shields PG, Freudenheim JL (2015) Associations of intakes of magnesium and calcium and survival among women with breast cancer: results from Western New York Exposures and breast cancer (WEB) Study. Am J Cancer Res 6(1):105–113
De La Cruz-Morcillo MA, García-Cano J, Arias-González L, García-Gil E, Artacho-Cordón F, Ríos-Arrabal S, Valero ML, Cimas FJ, Serrano-Oviedo L, Villas MV, Romero-Fernández J, Núñez MI, Sánchez-Prieto R (2013) Abrogation of the p38 MAPK a signaling pathway does not promote radioresistance but its activity is required for 5-fluorouracil-associated radiosensitivity. Cancer Lett 335(1):66–74
Murata A, Ito Y, Kashima R, Kanbayashi S, Nanatani K, Igarashi C, Okumura M, Inaba K, Tokino T, Takahashi S, Kamagata K (2015) One-dimensional sliding of p53 along DNA is accelerated in the presence of Ca(2+) or Mg(2+) at millimolar concentrations. J Mol Biol 427(16):2663–2678
Dhennin-Duthille I, Korichneva I, Ouadid-Ahidouch H (2014) TRPM7 involvement in cancer: a potential prognostic factor. Magnes Res 27(3):103–112
Hecht F, Pessoa CF, Gentile LB, Rosenthal D, Carvalho DP, Fortunato RS (2016) The role of oxidative stress on breast cancer development and therapy. Tumor Biol 37(4):4281–4291
Morais JBS, Severo JS, Santos LR, Melo SRS, Santos RO, Oliveira AR, Cruz KJC, Marreiro DN (2017) Role of magnesium in oxidative stress in individuals with obesity. Biol Trace Elem Res 176(1):20–26
Wolf FI, Trapani V (2012) Magnesium and its transporters in cancer a novel paradigm in tumour development. Clin Sci 123(7):417–427
Zhou L, Wang Y, Tian D, Yang J, Yang Y (2012) Decreased levels of nitric oxide production and nitric oxide synthase-2 expression are associated with the development and metastasis of hepatocellular carcinoma. Molecular Medicine Reports 6(6):1261–1266
Deepti R, Nalini G (2011) Magnesium plays a salient role in the cells. J Biomed Sci 4(4):341–345
Rocha VS, Rosa FBD, Ruano R, Zugaib M, Colli C (2015) Association between magnesium status, oxidative stress and inflammation in preeclampsia: a case control study. Clin Nutr 34(6):1166–1171
Kumaran RS, Choi Y, Singh V, Song H, Song K, Kim KJ, Kim HJ (2015) In vitro cytotoxic evaluation of MgO nanoparticles and their effect on the expression of ROS genes. Int J Mol Sci 16:7551–7564
Brasil. Ministério da Saúde. Resolução n°466/2012 (2012) Conselho Nacional de Pesquisa com Seres Humanos. Diário Oficial da União, Brasília
Taco. Tabela Brasileira de Composição de Alimentos. 4 ed. rev. ampl. Campinas: NEPA-UNICAMP, 2011. Disponível em: http://www.unicamp.br/nepa/taco/contar/taco_4_edicao_ampliada_e_revisada.pdf?arquivo=taco_4_versao_ampliada_e_revisada.pdf>. Acesso em 20 jan. 2017
Philippi ST (2002) Tabela de composição de alimentos: Suporte para decisão nutricional, 2nd edn. Coronário Editora, Brasília
USDA. United States Department of Agriculture. Agricultural Research Service. USDA National Nutrient Database for Standard Reference (2007) Release 20. U.S. Department of Agriculture, Riverdale, MD
Institute Of Medicine (1997) FOOD AND NUTRITION BOARD. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press, Washington, DC
Institute Of Medicine. FOOD AND NUTRITION BOARD. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (2005) Washington. National Academy Press, DC
Haubrock J, Nöthlings U, Volatier JL, Dekkers A, Ocké M, Harttig U, Illner AK, Knüppel S, Andersen LF, Boeing H, Efcoval C (2011) Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J Nutr 141(5):914–920
MSM. Multiple Source Method (MSM) for estimating usual dietary intake from short-term measurement data: user guide. EFCOVAL: Potsdam, 2011. 41p
Laureano GHC, Torman VB, Crispim SP, Dekkers AL, Camey SA (2016) Comparison of the ISU, NCI, MSM, and SPADE methods for estimating usual intake: a simulation study of nutrients consumed daily. Nutrients 8(3):166
Souverein OW, Dekkers AL, Geelen A, Haubrock J, De Vries JH, Ocké MC, Harttig U, Boeing H, Van’T Veer P (2011) EFCOVAL. Consortium. Comparing four methods to estimate usual intake distributions. European Journal of Clinical Nutrition 65(1):S92–S101
Beaton GH (1994) Approaches to analysis of dietary data: relationship between planned analyses and choice of methodology. Am J Clin Nutr 59(1):253–261
Elin RJ (1987) Assessment of magnesium status. Clin Chem 33(11):1965–1970
Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz. 3. ed. São Paulo: IMESP, 1985
Whitehouse RC, Prasad AS, Rabbani PI, Cossack ZT (1982) Zinc in plasma, neutrophils, lymphocytes, and erythrocytes as determined by flameless atomic absorption spectrophotometry. Clin Chem 28(3):475–480
Van Assendelft OW (1972) The measurement of hemoglobin. In: IZAK, G.; LEWIS, S. M. Modern Concepts in Hematology: symposia of the International Committee for Standardization in Hematology. New York: Academic Press
Topf JM, Murray PT (2003) Hypomagnesemia and hypermagnesemia. Reviews in Endocrine and Metabolic Disorders 4(2):195–206
Jahnen-Dechent W, Ketteler M (2012) Magnesium basics. Clin Kidney J 5(1):i3–i14
Tietz NW (1995) Clinical guide to laboratory test, 3rd edn. W.B. Saunders Company, Philadelphia
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Spickett CM, Wiswedel I, Siems W, Zarkovic K, Zarkovic N (2010) Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic Res 44(10):1172–1202
Mendes PMV, Bezerra DLC, Santos LR, Santos RO, Melo SRS, Morais JBS, Severo JS, Vieira SC, Marreiro DN (2018) Magnesium in breast cancer: what is its influence on the progression of this disease? Biol Trace Elem Res 184(2):334–339
Araújo MC, Bezerra IN, Barbosa FS, Junger WL, Yokoo EM, Pereira RAC, Sichieri R (2013) Consumo de macronutrientes e ingestão inadequada de micronutrientes em adultos. Rev Saude Publica 47(1):177S–189S
Surwillo A, Wawrzyniak A (2013) Nutritional assessment of selected patients with cancer. Roczniki Państwowego Zakładu Higieny 64(3):225–233
Severo JS, Morais JBS, Freitas TEC, Cruz KJC, Oliveira AR, Poltronieri F, Marreiro DN (2015) Aspectos metabólicos e nutricionais do magnésio. Nutrición Clínica y Dietética Hospitalaria 35(2):67–74
Huang WQ, Long WQ, Mo XF, Zhang NQ, Luo H, Lin FY, Huang J, Zhang CX (2019) Direct and indirect associations between dietary magnesium intake and breast cancer risk. Sci Rep 9:5764
Bradshaw PT, Khankari NK, Teitelbaum SL, Xu X, Fink BN, Steck SE, Gaudet MM, Kabat GC, Wolff MS, Neugut AI, Chen J, Gammon MD (2013) Nutrient pathways and breast cancer risk: the Long Island breast cancer study project. Nutr Cancer 65(3):345–354
Karki K, Pande D, Negi R, Khanna S, Khanna RS, Khanna HD (2015a) Association between biomarkers of oxidative stress, trace elements, and cell proliferation index in patients with benign and malignant breast diseases. J Environ Pathol Toxicol Oncol 34:1–10
Giménez-Mascarell P, Schirrmacher CE, Martínez-Cruz LA, Müller D (2018) Novel aspects of renal magnesium homeostasis. Front Pediatr 6(77):1–13
Araújo CGB, Holanda AON, Rocha CVS, Nascimento APS, Revoredo CMS, Silva BB, Nogueira NN, Marreiro DN (2015) Relationship between zincemia, superoxide dismutase activity and marker of oxidative stress in women with breast cancer. Nutr Hosp 32(2):785–791
Gupta RK, Patel AK, Kumari R, Chugh S, Shrivastav C, Mehra S, Sharma AN (2012) Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: a case control study. Asian Pac J Cancer Prev 13(12):6295–6298
Esrefoglu M (2012) Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon 12(3):160–167
Karki K, Pande D, Negi R, Khanna S, Khanna RS, Khanna HD (2014) Expression of serum toll-like receptor 9 and oxidative damage markers in benign and malignant breast diseases. DNA Cell Biol 33(9):630–636
Stark AA, Porat N, Volohonsky G, Komlosh A, Bluvshtein E, Tubi C, Steinberg P (2003) The role of gamma-glutamyl transpeptidase in the biosynthesis of glutathione. Biofactors 17(1–4):139–149
Niki E (2014) Biomarkers of lipid peroxidation in clinical material. Biochim Biophys Acta 1840(2):809–817
Funding
The authors declare no source of funding was available for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bezerra, D.L.C., Mendes, P.M.V., Melo, S.R.d. et al. Hypomagnesemia and Its Relationship with Oxidative Stress Markers in Women with Breast Cancer. Biol Trace Elem Res 199, 4466–4474 (2021). https://doi.org/10.1007/s12011-021-02579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02579-4