Abstract
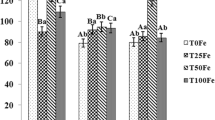
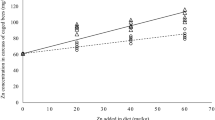
This study aimed to evaluate the quality of royal jelly produced by honeybees Apis mellifera supplemented with different concentrations of inorganic zinc (zinc sulfate monohydrate—0, 25, 50, and 75 ppm). Two-dimensional electrophoresis for the fractionation of royal jelly proteins was performed, and the zinc level was quantified by the flame atomic absorption spectrometry (FAAS) technique. Proteins were identified by electrospray ionization mass spectrometry (ESI MS MS). Analysis of variance followed by the Tukey test (P < 0.05) was used. Supplementation with the mineral zinc positively affected the quantification of proteins for treatments 50 and 75 ppm. However, all treatments independent of zinc concentrations showed fewer protein spots when compared to the control. All zinc-containing proteins were classified as major royal jelly proteins (MRJPs). The exposure of nursing bees to the mineral zinc in its inorganic form reduced the expression of six different MRJPs involved in larval and glands development of nursing bees (MRJP1, MRJP2, MRJP3, MRJP5, and MRJP7), however promoted an increase in the expression of royal jelly proteins involved in defense systems (MRJP8 and MRJP9). The results demonstrate that vital proteins and metabolic processes are impaired in nursing bees exposed to the mineral zinc in its inorganic form in all doses used affecting nutrition and maintenance of colonies.


Similar content being viewed by others
References
Lamontagne-Drolet M, Samson-Robert O, Giovenazzo P, Fournier V (2019) The impacts of two protein supplements on commercial honey bee (Apis mellifera) colonies. J Apic Res 58(5):800–813. https://doi.org/10.1080/00218839.2019.1644938
Ahmed ZH, Tawfik AI, Abdel-Rahman MF, Moustafa AM (2020) Nutritional value and physiological effects of some proteinaceous diets on honey bee workers (Apis mellifera L.). Bee World 97(1):26–31. https://doi.org/10.1080/0005772X.2019.1672983
Lin N, Chen S, Zhang H, Li J, Fu L (2018) Quantification of major royal jelly protein 1 in fresh royal jelly by ultraperformance liquid chromatography–tandem mass spectrometry. J Agric Food Chem 66(5):1270–1278. https://doi.org/10.1021/acs.jafc.7b05698
Ramanathan ANKG, Nair AJ, Sugunan VS (2018) A review on royal jelly proteins and peptides. J Funct Foods 44:255–264. https://doi.org/10.1016/j.jff.2018.03.008
Maghsoudlou A, Mahoonak AS, Mohebodini H, Toldra F (2019) Royal jelly: chemistry, storage and bioactivities. J Apicult Sci 63(1):17–40. https://doi.org/10.2478/jas-2019-0007
Dobritzsch D, Aumer D, Fuszard M, Erler S, Buttstedt A (2019) The rise and fall of major royal jelly proteins during a honeybee (Apis mellifera) workers’ life. Ecol Evol 9(15):8771–8782. https://doi.org/10.1002/ece3.5429
Pirk CWW (2018) Honeybee evolution: royal jelly proteins help queen larvae to stay on top. Curr Biol 28(8):R350–R351. https://doi.org/10.1016/j.cub.2018.02.065
Crailsheim K (1992) The flow of jelly within a honeybee colony. J Comp Physiol B 162(8):681–689. https://doi.org/10.1007/BF00301617
Wang Y, Ma L, Zhang W, Cui X, Wang H, Xu B (2016) Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 47(1):48–56. https://doi.org/10.1007/s13592-015-0374-x
Maori E, Navarro IC, Boncristiani H, Seilly DJ, Rudolph KLM, Sapetschnig A, Lin C, Ladbury JE, Evans JD, Heeney JH, Miska EA (2019) A secreted RNA binding protein forms RNA-stabilizing granules in the honeybee royal jelly. Mol Cell 74(3):598–608. https://doi.org/10.1016/j.molcel.2019.03.010
Scarselli R, Donadio E, Giuffrida MG, Fortunato D, Conti A, Balestreri E, Felicioli R, Pinzauti M, Sabatini AG (2005) Towards royal jelly proteome. Proteomics 5(3):769–776. https://doi.org/10.1002/pmic.200401149
Herbert EW Jr, Shimanuki H (1978) Chemical composition and nutritive value of bee collected and bee-stored pollen. Apidologie 9(1):33–40
Zhang G, Zhang W, Cui X, Xu B (2015) Zinc nutrition increases the antioxidant defenses of honey bees. Entomol Exp Appl 156(3):201–210. https://doi.org/10.1111/eea.12342
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41(3):278–294
De Paula Araújo WL, Negrão AF, Souza Vieira JC, Bittarello AC, De Magalhães Padilha P, De Oliveira Orsi R (2019) Supplementation with an inorganic iron source modulates the metalloproteomic profile of the royal jelly produced by Apis mellifera L. Biol Trace Elem Res:1–10. https://doi.org/10.1007/s12011-019-01863-8
Maret W (2017) Zinc in cellular regulation: The nature and significance of “zinc signals”. Int J Mol Sci 18(11):2285. https://doi.org/10.3390/ijms18112285
Abd El-Hack ME, Alagawany M, Chaudhry MT, Saeed M, Ahmad EAM, El-Sayed SAA (2020) Does the gradual increase in dietary zinc oxide supplementation can affect egg quality, serum indices, and productive performance of laying hens? Trop Anim Health Prod 52(2):525–531. https://doi.org/10.1007/s11250-019-02038-2
Xu X, Wang H, Li H, Sun H (2020) Metalloproteomic approaches for matching metals to proteins: the power of inductively coupled plasma mass spectrometry (ICP-MS). Chem Lett 49(X):697–704. https://doi.org/10.1246/cl.200155
Garcia JS, Magalhães CS, Arruda MAZ (2006) Trends in metal-binding and metalloprotein analysis. Talanta 69(1):1–15. https://doi.org/10.1016/j.talanta.2005.08.041
Brandão AR, Barbosa HS, Arruda MAZ (2010) Image analysis of two-dimensional gel electrophoresis for comparative proteomics of transgenic and non-transgenic soybean seeds. J Proteome 73:1433–1440. https://doi.org/10.1016/j.jprot.2010.01.009
Doolittle GMM (1899) Doolittlés queen rearing methods. Am Bee J 39(28):435–436
Braga CP, Bittarello AC, Padilha CCF, Leite AL, Moraes PM, Buzalaf MAR, Zara LF, Padilha PM (2015) Mercury fractionation in dourada (Brachyplatystoma rousseauxii) of the Madeira River in Brazil using metalloproteomic strategies. Talanta 32:239–244. https://doi.org/10.1016/j.talanta.2014.09.021
Vieira JC, Cavecci B, Queiroz JV, Braga CP, Padilha CC, Leite AL, Figueiredo W, Buzalaf M, Zara LF, Padilha PM (2015) Determination of the mercury fraction linked to protein of muscle and liver tissue of Tucunaré (Cichla spp.) from the Amazon Region of Brazil. Arch Environ Contam Toxicol 69(4):422–430. https://doi.org/10.1007/s00244-015-0160-9
Healthcare GE (2007) 2-D electrophoresis: principles and methods. GE Healthcare Limited
Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1(6):2856–2860. https://doi.org/10.1038/nprot.2006.468
Zar JH (2010) Bioestatistical analysis. Pretince Hall, New Jersey, p 944
Herbert EW Jr (2000) Honey bee nutrition. In: Graham J (ed) The hive and the honey bee. Dadant and Co., Hamilton, pp 197–224
Naug D (2009) Nutritional stress due to habitat loss may explain recent honeybee colony collapses. Biol Conserv 142(10):2369–2372. https://doi.org/10.1016/j.biocon.2009.04.007
Morais MM, Turcatto AP, Pereira RA, Francoy TM, Guidugli-lazzarini KR, Gonçalves LS, Almeida JMV, Ellis JD, De Jong D (2013) Protein levels and colony development of Africanized and European honey bees fed natural and artificial diets. Genet Mol Res 12(4):6915–6922. https://doi.org/10.4238/2013
Carrillo MP, Kadri SM, Veiga N, Orsi RO (2015) Energetic feedings influence beeswax production by Apis mellifera honeybees. Acta Scientiarum 37(1):73. https://doi.org/10.4025/actascianimsci.v37i1.24191
Eşanu D, Pop IM, Simeanu D (2018) The influence of some supplementary feeds on food consumption and bodyweight of caged honeybees. Anim Sci 51(1):15–20
Wright GA, Nicolson SW, Shafir S (2018) Nutritional physiology and ecology of honey bees. Annu Rev Entomol 63:327–344. https://doi.org/10.1146/annurev-ento-020117-043423
Chand N, Khan RU, Shah M, Naz S, Tinelli A (2020) Zinc source modulates zootechnical characteristics, intestinal features, humoral response, and paraoxonase (PON1) activity in broilers. Trop Anim Health Prod 52(2):511–515. https://doi.org/10.1007/s11250-019-02036-4
Fratini F, Cilia G, Mancini S, Felicioli A (2016) Royal jelly: an ancient remedy with remarkable antibacterial properties. Microbiol Res 192:130–141. https://doi.org/10.1016/j.micres.2016.06.007
Park MJ, Kim BY, Deng Y, Park HG, Choi YS, Lee KS, Jin BR (2020) Antioxidant capacity of major royal jelly proteins of honeybee (Apis mellifera) royal jelly. J Asia Pac Entomol 23(2):445–448. https://doi.org/10.1016/j.aspen.2020.03.007
Xun L, Huang X, Li Q, Yang S, Wang Y (2020) Effects of different bee pollens on expression of major royal jelly protein genes and yield, quality and composition of royal jelly of Apis mellifera. Chin J Anim Nutr 32(2):856–869
El-Guendouz S, Lyoussi B, Miguel MG (2020) Insight into the chemical composition and biological properties of Mediterranean royal jelly. J Apic Res 59:1–20. https://doi.org/10.1080/00218839.2020.1744241
Blank S, Bantleon FI, Mcintyre M, Oliert M, Spiliner, E (2012) The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin Exp Allergy 42:976-985. https://doi.org/10.1111/j.1365-2222.2012.03966.x
Funding
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), process 2018/00511-9.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001”.
Author information
Authors and Affiliations
Contributions
All authors have contributed equally to the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Almeida Longuini, A., Martineli, G.M., Camilli, M.P. et al. Supplementation with an Inorganic Zinc Source in the Metalloproteomic Profile of Royal Jelly in Apis mellifera L.. Biol Trace Elem Res 199, 4308–4318 (2021). https://doi.org/10.1007/s12011-020-02564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02564-3