Abstract
Maternal immune activation (MIA) model has been profoundly described as a suitable approach to study the pathophysiological mechanisms of neuropsychiatric disorders, including schizophrenia. Our previous study revealed that prenatal exposure to lipopolysaccharide (LPS) induced working memory impairments in only male offspring. Based on the putative role of prefrontal cortex (PFC) in working memory process, the current study was conducted to examine the long-lasting effect of LPS-induced MIA on several neuroinflammatory mediators in the PFC of adult male pups. We also investigated whether maternal zinc supplementation can alleviate LPS-induced alterations in this region. Pregnant rats received intraperitoneal injections of either LPS (0.5 mg/kg) or saline on gestation days 15/16 and supplemented with ZnSO4 (30 mg/kg) throughout pregnancy. At postnatal day 60, the density of both microglia and astrocyte cells and the expression levels of IL-6, IL-1β, iNOS, TNF-α, NF-κB, and GFAP were evaluated in the PFC of male pups. Although maternal LPS treatment increased microglia and astrocyte density, number of neurons in the PFC of adult offspring remained unchanged. These findings were accompanied by the exacerbated mRNA levels of IL-6, IL-1β, iNOS, TNF-α, NF-κB, and GFAP as well. Conversely, prenatal zinc supplementation alleviated the mentioned alterations induced by LPS. These findings support the idea that the deleterious effects of prenatal LPS exposure could be attenuated by zinc supplementation during pregnancy. It is of interest to suggest early therapeutic intervention as a valuable approach to prevent neurodevelopmental deficits, following maternal infection.
Graphical abstract

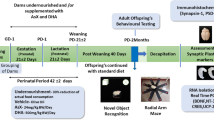
Schematic diagram describing the experimental timeline. On gestation days (GD) 15 and 16, pregnant dams were administered with intraperitoneal injections of either LPS (0.5 mg/kg) or vehicle and supplemented with ZnSO4 (30 mg/kg) throughout pregnancy by gavage. The resulting offspring were submitted to qPCR, immunostaining, and morphological analysis at PND 60. Maternal zinc supplementation alleviated increased expression levels of inflammatory mediators and microglia and astrocyte density induced by LPS in the PFC of treated offspring. PND postnatal day, PFC prefrontal cortex.





Similar content being viewed by others
Data Availability
The data that support the findings of this study can be accessed upon reasonable request from the corresponding author.
Abbreviations
- LPS:
-
Lipopolysaccharide
- PND:
-
Postnatal day
- PFC:
-
Prefrontal cortex
- GD:
-
Gestation day
- MIA:
-
Maternal immune activation
- CNS:
-
Central nervous system
- GFAP:
-
Glial fibrillary acidic protein
- TNF-α:
-
Tumor necrosis factor-alpha
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- NF-κB:
-
Nuclear factor kappa B
- iNOS:
-
Inducible nitric oxide synthase
- iba1:
-
Ionized calcium binding adaptor molecule 1
- DAPI:
-
4′,6-diamidino2-phenylindole
- MT:
-
Maternal metallothionein
- i.p.:
-
Intraperitoneal
- qPCR:
-
Quantitative PCR
- H&E:
-
Hematoxylin-eosin
- ANOVA:
-
Analysis of variance
- ELISA:
-
Enzyme-linked immunosorbent assay
References
McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW (2003) The neurodevelopmental hypothesis of schizophrenia: a review of recent developments. Ann Med 35(2):86–93
Lang UE, Puls I, Müller DJ, Strutz-Seebohm N, Gallinat J (2007) Molecular mechanisms of schizophrenia. Cell Physiol Biochem 20(6):687–702
Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES (2018) Maternal immune activation in neurodevelopmental disorders. Dev Dyn 247(4):588–619
Prata J, Santos SG, Almeida MI, Coelho R, Barbosa MA (2017) Bridging autism spectrum disorders and schizophrenia through inflammation and biomarkers-pre-clinical and clinical investigations. J Neuroinflammation 14(1):179
Bergdolt L, Dunaevsky A (2019) Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Prog Neurobiol 175:1–19
Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184
Santos-Toscano R, Borcel É, Ucha M, Orihuel J, Capellán R, Roura-Martínez D, Ambrosio E, Higuera-Matas A (2016) Unaltered cocaine self-administration in the prenatal LPS rat model of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 69:38–48
Waterhouse U, Roper VE, Brennan KA, Ellenbroek BA (2016) Nicotine ameliorates schizophrenia-like cognitive deficits induced by maternal LPS exposure: a study in rats. Dis Model Mech 9(10):1159–1167
Wischhof L, Irrsack E, Osorio C, Koch M (2015) Prenatal LPS-exposure–a neurodevelopmental rat model of schizophrenia–differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog Neuro-Psychopharmacol Biol Psychiatry 57:17–30
Alizadeh F, Davoodian N, Kazemi H, Ghasemi-Kasman M, Shaerzadeh F (2020) Prenatal zinc supplementation attenuates lipopolysaccharide-induced behavioral impairments in maternal immune activation model. Behav Brain Res 377:112247
O’Loughlin E, Pakan JM, Yilmazer-Hanke D, McDermott KW (2017) Acute in utero exposure to lipopolysaccharide induces inflammation in the pre-and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation 14(1):212
Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL (2000) Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 48(2):99–109
Perlstein WM, Carter CS, Noll DC, Cohen JD (2001) Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatr 158(7):1105–1113
Ruiz S, Birbaumer N, Sitaram R (2013) Abnormal neural connectivity in schizophrenia and fMRI-brain-computer interface as a potential therapeutic approach. Front Psychiatry 4:17
Bernstein H-G, Steiner J, Guest PC, Dobrowolny H, Bogerts B (2015) Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res 161(1):4–18
De Keyser J, Mostert JP, Koch MW (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 267(1–2):3–16
Lanz TA, Reinhart V, Sheehan MJ, Rizzo SJS, Bove SE, James LC, Volfson D, Lewis DA, Kleiman RJ (2019) Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: a comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl Psychiatry 9(1):1–13
Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S (2013) Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuro-Psychopharmacol Biol Psychiatry 42:115–121
Juckel G, Manitz MP, Brüne M, Friebe A, Heneka MT, Wolf RJ (2011) Microglial activation in a neuroinflammational animal model of schizophrenia—a pilot study. Schizophr Res 131(1–3):96–100
Radewicz K, Garey LJ, Gentleman SM, Reynolds R (2000) Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 59(2):137–150
Schnieder TP, Dwork AJ (2011) Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry 69(2):134–139
Matute C, Melone M, Vallejo-Illarramendi A, Conti F (2005) Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia 49(3):451–455
Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD (2015) The poly (I: C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149:213–226
Yu X, Chen W, Wei Z, Ren T, Yang X, Yu X (2016) Effects of maternal mild zinc deficiency and different ways of zinc supplementation for offspring on learning and memory. Food Nutr Res 60(1):29467
Carey LC, Berbée PL, Coyle P, Philcox JC, Rofe AM (2003) Zinc treatment prevents lipopolysaccharide-induced teratogenicity in mice. Birth Defects Res A: Clin Molec Teratol 67(4):240–245
Kirsten TB, Chaves-Kirsten GP, Bernardes S, Scavone C, Sarkis JE, Bernardi MM, Felicio LF (2015) Lipopolysaccharide exposure induces maternal hypozincemia, and prenatal zinc treatment prevents autistic-like behaviors and disturbances in the striatal dopaminergic and mTOR systems of offspring. PLoS One 10(7):e0134565
Levenson CW, Morris D (2011) Zinc and neurogenesis: making new neurons from development to adulthood. Adv Nutr 2(2):96–100
Chua JS, Cowley CJ, Manavis J, Rofe AM, Coyle P (2012) Prenatal exposure to lipopolysaccharide results in neurodevelopmental damage that is ameliorated by zinc in mice. Brain Behav Immun 26(2):326–336
Coyle P, Tran N, Fung JN, Summers BL, Rofe AM (2009) Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res 197(1):210–218
Sharma N, Arora P, Nehru B (2017) Prenatal zinc supplementation to lipopolysaccharide infected female rats prevents neurochemical, behavioral and biochemical deficits produced in infants. Neuroimmunol Neuroinflamm 4(4):33–45
Chua JS, Rofe AM, Coyle P (2006) Dietary zinc supplementation ameliorates LPS-induced teratogenicity in mice. Pediatr Res 59(3):355–358
Moazedi A, Ghotbeddin Z, Parham G (2007) Comparison of the effects of dose-dependent zinc chloride on short-term and long-term memory in young male rats. Pak J Biol Sci 10(16):2704–2708
Charan J, Kantharia N (2013) How to calculate sample size in animal studies? J Pharmacol Pharmacother 4(4):303–306
Chiu K, Lau WM, Lau HT, So K-F, Chang RC-C (2007) Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Visual Exp 7:269
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL (2009) The MIQE guidelines: minimum information for publication of quantitative Real-Time PCR experiments. Clin Chem 55(4):611–622
Naeimi R, Safarpour F, Hashemian M, Tashakorian H, Ahmadian SR, Ashrafpour M, Ghasemi-Kasman M (2018) Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci Lett 674:1–10
Akbari A, Khalili-Fomeshi M, Ashrafpour M, Moghadamnia AA, Ghasemi-Kasman M (2018) Adenosine A2A receptor blockade attenuates spatial memory deficit and extent of demyelination areas in lyolecithin-induced demyelination model. Life Sci 205:63–72
Kirkpatrick B, Miller BJ (2013) Inflammation and schizophrenia. Schizophr Bull 39(6):1174–1179
Luo Y, He H, Zhang J, Ou Y, Fan N (2019) Changes in serum TNF-α, IL-18, and IL-6 concentrations in patients with chronic schizophrenia at admission and at discharge. Compr Psychiatry 90:82–87
Mazza MG, Lucchi S, Rossetti A, Clerici M (2020) Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in nonaffective psychosis: A meta-analysis and systematic review. World J Biol Psychiatry 21(5):326–338
Miller BJ, Culpepper N, Rapaport MH (2014) C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses 7(4):223–230
Wang AK, Miller BJ (2018) Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull 44(1):75–83
Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013) Maternal immune activation causes age-and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31:54–68
Ding S, Hu Y, Luo B, Cai Y, Hao K, Yang Y, Zhang Y, Wang X, Ding M, Zhang H (2019) Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with poly I: C in early gestation. Behav Brain Funct 15(1):3
Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 25(1):11–24
Paylor JW, Lins BR, Greba Q, Moen N, De Moraes RS, Howland JG, Winship IR (2016) Developmental disruption of perineuronal nets in the medial prefrontal cortex after maternal immune activation. Sci Rep 6:37580
Li X, Tian X, Lv L, Hei G, Huang X, Fan X, Zhang J, Zhang J, Pang L, Song X (2018) Microglia activation in the offspring of prenatal poly I: C exposed rats: a PET imaging and immunohistochemistry study. Gen Psychiatry 31(1):e000006
Hao L, Hao X, Li S, Li X (2010) Prenatal exposure to lipopolysaccharide results in cognitive deficits in age-increasing offspring rats. Neuroscience 166(3):763–770
Fields RD, Woo DH, Basser PJ (2015) Glial regulation of the neuronal connectome through local and long-distant communication. Neuron 86(2):374–386
Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, Suzuki M (2012) Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res Neuroimaging 202(3):233–238
Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P (2003) Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage 19(3):751–763
Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM (2005) Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain 128(9):2097–2108
Portbury SD, Adlard PA (2017) Zinc signal in brain diseases. Int J Mol Sci 18(12):2506
Cai L, Chen T, Yang J, Zhou K, Yan X, Chen W, Sun L, Li L, Qin S, Wang P (2015) Serum trace element differences between schizophrenia patients and controls in the Han Chinese population. Sci Rep 5:15013
Cao B, Yan L, Ma J, Jin M, Park C, Nozari Y, Kazmierczak OP, Zuckerman H, Lee Y, Pan Z (2019) Comparison of serum essential trace metals between patients with schizophrenia and healthy controls. J Trace Elem Med Biol 51:79–85
Joe P, Petrilli M, Malaspina D, Weissman J (2018) Zinc in schizophrenia: a meta-analysis. Gen Hosp Psychiatry 53:19–24
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651
Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85(3):837–844
von Bülow V, Dubben S, Engelhardt G, Hebel S, Plümäkers B, Heine H, Rink L, Haase H (2007) Zinc-dependent suppression of TNF-α production is mediated by protein kinase A-induced inhibition of Raf-1, IκB kinase β, and NF-κB. J Immunol 179(6):4180–4186
Ghosh S, Hayden MS (2012) Celebrating 25 years of NF-κB research. Immunol Rev 246(1):5–13
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:14
Funding
This study was supported by a grant [grant no. 960329] from the Research Vice-Chancellor of Hormozgan University for Medical Science (HUMS).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: Nahid Davoodian and Fatemeh Shaerzadeh
Data collection: Ronak Mousaviyan, Faezeh Alizadeh, Nahid Davoodian, and Haniyeh Kazemi
Data analysis: Nahid Davoodian, Maryam Ghasemi-Kasman, and Seyed Abdollah Mousavi
Manuscript preparation: Nahid Davoodian and Maryam Ghasemi-Kasman
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interest.
Ethics Approval
All animal procedures in this research were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and also approved by the local Institutional Ethics Committee (approval number: IR.HUMS.REC.1397.010).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mousaviyan, R., Davoodian, N., Alizadeh, F. et al. Zinc Supplementation During Pregnancy Alleviates Lipopolysaccharide-Induced Glial Activation and Inflammatory Markers Expression in a Rat Model of Maternal Immune Activation. Biol Trace Elem Res 199, 4193–4204 (2021). https://doi.org/10.1007/s12011-020-02553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02553-6