Abstract
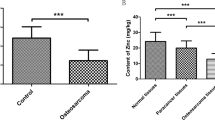
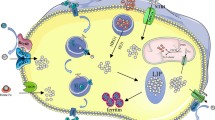
Zinc is a trace element in human body involved in many biological processes. It is critical for cell growth and acts as a cofactor for the structure and function of a wide range of cellular proteins such as enzymes. Mounting evidence has shown the involvement of intracellular zinc in the bone-related biological processes such as bone growth, homeostasis, and regeneration; however, the molecular mechanism(s) whereby zinc impels tumorigenesis in bone remains largely unexplored. In this article, selective outline related to the content of intracellular zinc in osteosarcoma cells was provided, and its correlation with signaling molecules that are activated and consequently guide the cells toward tumorigenesis or osteogenesis was discussed. Based on preclinical and clinical evidence, dysregulation of zinc homeostasis, both at intracellular and tissue level, has the main role in the pathogenesis of osteosarcoma. Based on the intracellular zinc content, this element could have a direct role in the dynamics of bone cell transformation and tumor development and play an indirect role in the modulation of the inflammatory and pro/antitumorigenic responses in immune cells. In this context, zinc transporters and the proteins containing zinc domain are regulated by the availability of zinc, playing a crucial role in bone cell transformation and differentiation. According to recent studies, it seems that intracellular zinc levels could be considered as an early prognosis marker. Besides, identification and targeting of zinc-dependent signaling molecules could tilt the balance of life and death toward the latter in chemoresistant malignant cells and may pave a way for designing of the novel osteosarcoma treatment strategies.




Similar content being viewed by others
References
He H, Ni J, Huang J (2014) Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett 7(5):1352–1362
Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koefflar HP (1996) Alterations of thep53, Rb andMDM2 genes in osteosarcoms. J Cancer Res Clin Oncol 122(9):559–565
Rejniak KA, Lloyd MC, Reed DR, Bui MM (2015) Diagnostic assessment of osteosarcoma chemoresistance based on Virtual Clinical Trials. Med Hypotheses 85(3):348–354
Krężel A, Maret W (2006) Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 11(8):1049–1062
Maret W (2015) Analyzing free zinc (ii) ion concentrations in cell biology with fluorescent chelating molecules. Metallomics 7(2):202–211
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118
Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T (2007) Zinc is a novel intracellular second messenger. J Cell Biol 177(4):637–645
Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T (2011) Zinc homeostasis and signaling in health and diseases. J Biol Inorg Chem 16(7):1123–1134
Murakami M, Hirano T (2008) Intracellular zinc homeostasis and zinc signaling. Cancer Sci 99(8):1515–1522
Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T (2006) Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7(9):971–977
Yamashita S, Miyagi C, Fukada T, Kagara N, Che Y-S, Hirano T (2004) Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature 429(6989):298–302
Zhang N, Duncan FE, Que EL, O’Halloran TV, Woodruff TK (2016) The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci Rep 6:22772
Yu M, Lee W-W, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ (2011) Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med 208(4):775–785
O'Connor JP, Kanjilal D, Teitelbaum M, Lin SS, Cottrell JA (2020) Zinc as a therapeutic agent in bone regeneration. Materials (Basel) 13(10):2211
Amin N, Clark CCT, Taghizadeh M, Djafarnejad S (2020) Zinc supplements and bone health: The role of the RANKL-RANK axis as a therapeutic target. J Trace Elem Med Biol 57:126417
Chen D, Waite LC, Pierce WM (1998) In vitro bone resorption is dependent on physiological concentrations of zinc. Biol Trace Elem Res 61(1):9–18
Sun J-Y, Wang J-F, Zi N-T, Jing M-Y, Weng X-Y (2011) Effects of zinc supplementation and deficiency on bone metabolism and related gene expression in rat. Biol Trace Elem Res 143(1):394–402
Yamaguchi M (2010) Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Bioch 338(1-2):241–254
Park JH, Grandjean CJ, Antonson DL, Vanderhoof JA (1986) Effects of isolated zinc deficiency on the composition of skeletal muscle, liver and bone during growth in rats. J Nutr 116(4):610–617
Windisch W, Wher U, Rambeck W, Erben R (2002) Effect of Zn deficiency and subsequent Zn repletion on bone mineral composition and markers of bone tissue metabolism in 65Zn-labelled, young-adult rats. J Anim Physiol Anim Nutr 86(7-8):214–221
Gurban CV, Mederle O (2011) The OPG/RANKL system and zinc ions are promoters of bone remodeling by osteoblast proliferation in postmenopausal osteoporosis. Romanian journal of morphology and embryology. Rev Roum Morphol Embryol 52(3 Suppl):1113–1119
Park KH, Choi Y, Yoon DS, Lee KM, Kim D, Lee JW (2018) Zinc promotes osteoblast differentiation in human mesenchymal stem cells via activation of the cAMP-PKA-CREB signaling pathway. Stem Cells Dev 27(16):1125–1135
Meshkini A, Oveisi H (2017) Methotrexate-F127 conjugated mesoporous zinc hydroxyapatite as an efficient drug delivery system for overcoming chemotherapy resistance in osteosarcoma cells. Colloids Surf B 158:319–330
Atkins GJ, Findlay DM, Anderson PH, Morris HA (2011) Chapter 23 - target genes: bone proteins. In: Feldman D, Pike JW, Adams JS (eds) Vitamin D, 3rd edn. Academic Press, San Diego, pp 411–424
Chen D, Waite LC, Pierce WM (1999) In vitro effects of zinc on markers of bone formation. Biol Trace Elem Res 68(3):225–234
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Ann Rev Biophysics Biomol Struct 21:441–483
Hove E, Elvehjem C, Hart E (1940) The effect of zinc on alkaline phosphatases. J Biol Chem 134:425
Ferreira ECS, Bortolin RH, Freire-Neto FP, Souza KSC, Bezerra JF, Ururahy MAG, Ramos AMO, Himelfarb ST, Abreu BJ, Didone TVN, Pedrosa LFC, Medeiros AC, Doi SQ, Brandão-Neto J, Hirata RDC, Rezende LA, Almeida MG, Hirata MH, Rezende AA (2017) Zinc supplementation reduces RANKL/OPG ratio and prevents bone architecture alterations in ovariectomized and type 1 diabetic rats. Nutr Res 40:48–56
Liu W, Zhang X (2015) Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review). Mol Med Rep 11(5):3212–3218
Wu X, Li F, Dang L, Liang C, Lu A, Zhang G (2020) RANKL/RANK system-based mechanism for breast cancer bone metastasis and related therapeutic strategies. Front Cell Dev Biol 8:76
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S (2020) RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. BioMed Res Int 2020:6910312
Ono T, Hayashi M, Sasaki F, Nakashima T (2020) RANKL biology: bone metabolism, the immune system, and beyond. Inflammation Regener 40(1):2
Ardeshirpour L, Dumitru C, Dann P, Sterpka J, VanHouten J, Kim W, Kostenuik P, Wysolmerski J (2015) OPG treatment prevents bone loss during lactation but does not affect milk production or maternal calcium metabolism. Endocrinology 156(8):2762–2773
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12(9):1260–1268
Hie M, Tsukamoto I (2011) Administration of zinc inhibits osteoclastogenesis through the suppression of RANK expression in bone. Eur J Pharmacol 668(1):140–146
Fong L, Tan K, Tran C, Cool J, Scherer MA, Elovaris R, Coyle P, Foster BK, Rofe AM, Xian CJ (2009) Interaction of dietary zinc and intracellular binding protein metallothionein in postnatal bone growth. Bone 44(6):1151–1162
Fushimi H, Inoue T, Yamada Y, Horie H, Kameyama M, Inoue K, Minami T, Okazaki Y (1993) Zinc deficiency exaggerates diabetic osteoporosis. Diabetes Res Clin Pract 20(3):191–196
Qi S, He J, Zheng H, Chen C, Jiang H, Lan S (2020) Zinc supplementation increased bone mineral density, improves bone histomorphology, and prevents bone loss in diabetic rat. Biol Trace Elem Res 194(2):493–501
Bortolin RH, da Graça Azevedo Abreu BJ, Abbott Galvão Ururahy M, Costa de Souza KS, Bezerra JF, Loureiro MB et al (2015) Protection against T1DM-induced bone loss by zinc supplementation: biomechanical, histomorphometric, and molecular analyses in STZ-induced diabetic rats. PloS One 10(5):e0125349-e
Iitsuka N, Hie M, Tsukamoto I (2013) Zinc supplementation inhibits the increase in osteoclastogenesis and decrease in osteoblastogenesis in streptozotocin-induced diabetic rats. Eur J Pharmacol 714(1-3):41–47
Zheng H, Qi S, Chen C (2018) Salidroside improves bone histomorphology and prevents bone loss in ovariectomized diabetic rats by upregulating the OPG/RANKL ratio. Molecules 23(9):2398
Nimmanon T, Taylor KM (2019) Post-translational mechanisms of zinc signalling in cancer. In: Fukada T, Kambe T (eds) Zinc Signaling. Springer Singapore, Singapore, pp 319–345
Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, Hrabeta J, Eckschlager T, Stiborova M, Kizek R (2011) Insight to physiology and pathology of zinc (II) ions and their actions in breast and prostate carcinoma. Curr Med Chem 18(33):5041–5051
Huang T, Yan G, Guan M (2020) Zinc homeostasis in bone: zinc transporters and bone diseases. Int J Mol Sci 21(4):1236
Alam S, Kelleher SLJN (2012) Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 4(8):875–903
Skrajnowska D, Bobrowska-Korczak B (2019) Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 11(10):2273
Mulay IL, Roy R, Knox BE, Suhr NH, Delaney WE (1971) Trace-metal analysis of cancerous and non-cancerous human tissues2. J Natl Cancer Inst 47(1):1–13
Bafaro E, Liu Y, Xu Y, Dempski RE (2017) The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct Target Ther 2:17029
Costello LC, Franklin RB (2006) The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer 5:17
To PK, Do MH, Cho J-H, Jung C (2020) Growth modulatory role of zinc in prostate cancer and application to cancer therapeutics. Int J Mol Sci 21(8):2991
Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ (2012) The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol 30(6):906–911
Han CT, Schoene NW, Lei KY (2009) Influence of zinc deficiency on Akt-Mdm2-p53 and Akt-p21 signaling axes in normal and malignant human prostate cells. Am J Physiology Cell Physiol 297(5):C1188–C1199
Gao K, Zhang Y, Niu J, Nie Z, Liu Q, Lv C (2020) Zinc promotes cell apoptosis via activating the Wnt-3a/β-catenin signaling pathway in osteosarcoma. J Orthopaedic Surg Res 15(1):57
Ziliotto S, Ogle O, Taylor KM (2018) Targeting zinc(II) signalling to prevent cancer. Met Ions life Sci 18:507–530
Behnamsani A, Meshkini A (2019) Synthesis and engineering of mesoporous ZnO@HAP heterostructure as a pH-sensitive nano-photosensitizer for chemo-photodynamic therapy of malignant tumor cells. J Drug Delivery Sci Technol 53:101200
Cerovic A, Miletic I, Sobajic S, Blagojevic D, Radusinovic M, El-Sohemy A (2007) Effects of zinc on the mineralization of bone nodules from human osteoblast-like cells. Biol Trace Elem Res 116(1):61–71
Gutiérrez MF, Alegría-Acevedo LF, Méndez-Bauer L, Bermudez J, Dávila-Sánchez A, Buvinic S, Hernández-Moya N, Reis A, Loguercio AD, Farago PV, Martin J, Fernández E (2019) Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J Dent 82:45–55
Begam H, Kundu B, Chanda A, Nandi SK (2017) MG63 osteoblast cell response on Zn doped hydroxyapatite (HAp) with various surface features. Ceram Int 43(4):3752–3760
Tsukahara T, Nabeta Y, Kawaguchi S, Ikeda H, Sato Y, Shimozawa K, Ida K, Asanuma H, Hirohashi Y, Torigoe T, Hiraga H, Nagoya S, Wada T, Yamashita T, Sato N (2004) Identification of human autologous cytotoxic T-lymphocyte-defined osteosarcoma gene that encodes a transcriptional regulator, papillomavirus binding factor. Cancer Res 64(15):5442–5448
Endo-Munoz L, Cumming A, Sommerville S, Dickinson I, Saunders NA (2010) Osteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal bone. Br J Cancer 103(1):73–81
Habel N, Hamidouche Z, Girault I, Patiño-García A, Lecanda F, Marie PJ et al (2013) Zinc chelation: a metallothionein 2A’s mechanism of action involved in osteosarcoma cell death and chemotherapy resistance. Cell Death Dis 4(10):e874-e
Krizkova S, Masarik M, Majzlik P, Kukacka J, Kruseova J, Adam V, Prusa R, Eckschlager T, Stiborova M, Kizek R (2010) Serum metallothionein in newly diagnosed patients with childhood solid tumours. Acta biochim Pol 57(4):561–566
Si M, Lang J (2018) The roles of metallothioneins in carcinogenesis. J Hematol Oncol 11(1):107
Eckschlager T, Adam V, Hrabeta J, Figova K, Kizek R (2009) Metallothioneins and cancer. Curr Protein Pept Sci 10(4):360–375
Abdel-Mageed AB, Agrawal KC (1998) Activation of nuclear factor kappaB: potential role in metallothionein-mediated mitogenic response. Cancer Res 58(11):2335–2338
Ostrakhovitch EA, Olsson P-E, Jiang S, Cherian MG (2006) Interaction of metallothionein with tumor suppressor p53 protein. FEBS Lett 580(5):1235–1238
Guo W, Zhao YP, Jiang YG, Wang RW, Hong L, Fan DM (2008) ZNRD1 might mediate UV irradiation related DNA damage and repair in human esophageal cancer cells by regulation of ERCC1. Dis Esophagus 21(8):730–736
Hong L, Chen Z, Zhang X, Xia L, Han Z, Lu Y, Jin H, Song J, Qiao T, Fan D (2006) Zinc ribbon domain containing 1 protein: modulator of multidrug resistance, tumorigenesis and cell cycle. Exp Oncol 28(4):258–262
Xie B, Li Y, Zhao R, Xu Y, Wu Y, Wang J et al (2018) Identification of key genes and miRNAs in osteosarcoma patients with chemoresistance by bioinformatics analysis. BioMed Res Int 2018:4761064
Hong L, Qiao T, Han Y, Han S, Zhang X, Lin T, Gao J, Zhao P, Chen Z, Fan D (2006) ZNRD1 mediates resistance of gastric cancer cells to methotrexate by regulation of IMPDH2 and Bcl-2. Biochemistry and cell biology. Biochim Biol Cell 84(2):199–206
Hong L, Piao Y, Han Y, Wang J, Zhang X, Du Y et al (2005) Zinc ribbon domain-containing 1 (ZNRD1) mediates multidrug resistance of leukemia cells through regulation of P-glycoprotein and Bcl-2. Mol Cancer Ther 4(12):1936–1942
Fellenberg J, Kunz P, Sähr H, Depeweg D (2010) Overexpression of inosine 5'-monophosphate dehydrogenase type II mediates chemoresistance to human osteosarcoma cells. PloS One 5(8):e12179
Kambe T, Suzuki E, Komori T (2019) Zinc transporter proteins: a review and a new view from biochemistry. In: Fukada T, Kambe T (eds) Zinc Signaling. Singapore, Springer Singapore p, pp 23–56
Pan Z, Choi S, Ouadid-Ahidouch H, Yang J-M, Beattie JH, Korichneva I (2017) Zinc transporters and dysregulated channels in cancers. Front Biosci (Landmark Ed) 22:623–643
Takatani-Nakase T (2018) Zinc Transporters and the Progression of Breast Cancers. Biol Pharm Bull 41(10):1517–1522
Franz MC, Anderle P, Bürzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G (2013) Zinc transporters in prostate cancer. Mol Aspects Med 34(2):735–741
Jin J, Li Z, Liu J, Wu Y, Gao X, He Y (2015) Knockdown of zinc transporter ZIP5 (SLC39A5) expression significantly inhibits human esophageal cancer progression. Oncol Rep 34(3):1431–1439
Tang Z, Sahu SN, Khadeer MA, Bai G, Franklin RB, Gupta A (2006) Overexpression of the ZIP1 zinc transporter induces an osteogenic phenotype in mesenchymal stem cells. Bone 38(2):181–198
Fu X, Li Y, Huang T, Yu Z, Ma K, Yang M et al (2018) Runx2/osterix and zinc uptake synergize to orchestrate osteogenic differentiation and citrate containing bone apatite formation. Adv Sci 5(4):1700755
Costello LC, Chellaiah MA, Zou J, Reynolds MA, Franklin RB (2015) In vitro BMP2 stimulation of osteoblast citrate production in concert with mineralized bone nodule formation. J Regener Med Tissue Eng 4:2
Yu Y, Liu K, Wen Z, Liu W, Zhang L, Su J (2020) Double-edged effects and mechanisms of Zn2+ microenvironments on osteogenic activity of BMSCs: osteogenic differentiation or apoptosis. RSC Adv 10(25):14915–14927
Kim G, Elnabawi O, Shin D, E-KJFip P (2016) Transient intermittent hypoxia exposure disrupts neonatal bone strength. Front Pediatr 4:15
Sasaki S, Tsukamoto M, Saito M, Hojyo S, Fukada T, Takami M, Furuichi T (2018) Disruption of the mouse Slc39a14 gene encoding zinc transporter ZIP14 is associated with decreased bone mass, likely caused by enhanced bone resorption. FEBS Open Bio 8(4):655–663
Suzuki T, Katsumata S, Matsuzaki H, Suzuki K (2016) A short-term zinc-deficient diet decreases bone formation through down-regulated BMP2 in rat bone. Biosci Biotechnol Biochem 80(7):1433–1435
Liu Y, Yan F, Yang WL, Lu XF, Wang WB (2013) Effects of zinc transporter on differentiation of bone marrow mesenchymal stem cells to osteoblasts. Biol Trace Elem Res 154(2):234–243
Alluri K, Nair KPM, Kotturu SK, Ghosh S (2020) Transcriptional regulation of zinc transporters in human osteogenic sarcoma (Saos-2) cells to zinc supplementation and zinc depletion. Biol Trace Elem Re 194(2):360–367
Zhang L-C, Chen L-Y (2019) A review on biomedical titanium alloys: recent progress and prospect. Adv Eng Mater 21(4):1801215
Smolle MA, Andreou D, Tunn P-U, Leithner A (2019) Advances in tumour endoprostheses: a systematic review. EFORT Open Rev 4(7):445–459
Chouirfa H, Bouloussa H, Migonney V, Falentin-Daudré C (2019) Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater 83:37–54
Eyvazi S, Vostakolaei MA, Dilmaghani A, Borumandi O, Hejazi MS, Kahroba H, Tarhriz V (2020) The oncogenic roles of bacterial infections in development of cancer. Microb Pathog 141:104019
Agassandian M, Shurin GV (2015) Bacterial infections and cancer development. In: Shurin MR, Thanavala Y, Ismail N (eds) Infection and Cancer: Bi-Directorial Interactions. Springer International Publishing p, Cham, pp 49–74
Gao C, Li C, Wang C, Qin Y, Wang Z, Yang F, Liu H, Chang F, Wang J (2017) Advances in the induction of osteogenesis by zinc surface modification based on titanium alloy substrates for medical implants. J Alloys Compd 726:1072–1084
Fernandes MH, Alves MM, Cebotarenco M, Ribeiro IAC, Grenho L, Gomes PS, Carmezim MJ, Santos CF (2020) Citrate zinc hydroxyapatite nanorods with enhanced cytocompatibility and osteogenesis for bone regeneration. Mater Sci Eng C 115:111147
Ortiz IY, Raybolt dos Santos A, Costa AM, Mavropoulos E, Tanaka MN, Prado da Silva MH, de Souza Camargo S Jr (2016) In vitro assessment of zinc apatite coatings on titanium surfaces. Ceram Int 42(14):15502–15510
Valanezhad A, Tsuru K, Maruta M, Kawachi G, Matsuya S, Ishikawa K (2012) A new biocompatible coating layer applied on titanium substrates using a modified zinc phosphatizing method. Surf Coat Technol 206(8):2207–2212
Li G, Niu L, Lian J, Jiang Z (2004) A black phosphate coating for C1008 steel. Surf Coat Technol 176(2):215–221
Zhong Z, Qin J, Ma J (2015) Electrophoretic deposition of biomimetic zinc substituted hydroxyapatite coatings with chitosan and carbon nanotubes on titanium. Ceram Int 41(7):8878–8884
Yang M, Shuai Y, Zhang C, Chen Y, Zhu L, Mao C, OuYang H (2014) Biomimetic nucleation of hydroxyapatite crystals mediated by Antheraea pernyi silk sericin promotes osteogenic differentiation of human bone marrow derived mesenchymal stem cells. Biomacromolecules. 15:1185–1193
Yang Y, Tao B, Gong Y, Chen R, Yang W, Lin C, Chen M, Qin L, Jia Y, Cai K (2020) Functionalization of Ti substrate with pH-responsive naringin-ZnO nanoparticles for the reconstruction of large bony after osteosarcoma resection. J Biomed Mater Res Part A 108(11):2190–2205
Saxena V, Hasan A, Pandey LM (2018) Effect of Zn/ZnO integration with hydroxyapatite: a review. Mater Technol 33(2):79–92
Zhao S, Xu Y, Xu W, Weng Z, Cao F, Wan X, Cui T, Yu Y, Liao L, Wang X (2020) Tremella-like ZnO@Col-I-decorated titanium surfaces with dual-light-defined broad-spectrum antibacterial and triple osteogenic properties. ACS Appl Mater Interfaces 12(27):30044–30051
Funding
The authors appreciate the financial support for this investigation by the Research Council of Ferdowsi University of Mashhad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meshkini, A. A Correlation Between Intracellular Zinc Content and Osteosarcoma. Biol Trace Elem Res 199, 3222–3231 (2021). https://doi.org/10.1007/s12011-020-02466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02466-4