Abstract
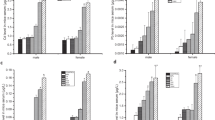
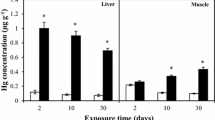
Mercury chloride (HgCl2) is a compound found in the environment that presents low risk due to low liposolubility. Considering the importance of blood as access rout to the systemic distribution of this toxicant to the organism as well as functions performed by it, this study aimed to investigate the effects of HgCl2 on the peripheral blood of rats, evaluating the oxidative biochemistry, blood count, and morphology of cell populations. For this, 20 adult Wistar male rats were divided into control (n = 10) and exposed (n = 10) groups and received distilled water or HgCl2 at a dose of 0.375 mg/kg for 45 days, respectively, through intragastric gavage. Then, the animals were euthanized and the blood was collected for total mercury (Hg) levels determination, complete blood and reticulocyte count, oxidative biochemistry by Trolox Equivalent Antioxidant Capacity (TEAC), reduced glutathione (GSH) levels, superoxide dismutase activity (SOD), thiobarbituric acid reactive substances (TBARS), and nitric oxide (NO), in blood cells and plasma. Long-term exposure increased total Hg in plasma and blood cells. In blood cells, only TEAC has decreased; in plasma, the HgCl2 increased TBARS and NO levels, followed by a decrease in TEAC and GSH levels. There were no quantitative changes in reticulocytes, erythrocytes, and hemoglobin; however, the number of leukocytes have increased and platelets have decreased. Our results suggest that even in the face of low toxicity when compared with other mercury species, HgCl2 at low doses is able to modulate the systemic redox balance and affect some blood cell populations.




Similar content being viewed by others
References
Organization, W.H. (2008) Guidance for identifying populations at risk from mercury exposure
Bernhoft RA (2012) Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012:1–10
Franco JL, Braga Hde C, Nunes AK, Ribas CM, Stringari J, Silva AP, Garcia Pomblum SC, Moro AM, Bohrer D, Santos AR, Dafre AL, Farina M (2007) Lactational exposure to inorganic mercury: evidence of neurotoxic effects. Neurotoxicol Teratol 29(3):360–367
Azimi S, Moghaddam M (2013) Effect of mercury pollution on the urban environment and human health. Environ Ecol Res 1(1):12–20
Park J-D, Zheng W (2012) Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health 45(6):344
Takahashi T, Shimohata T (2019) Vascular dysfunction induced by mercury exposure. Int J Mol Sci 20(10):2435
Omanwar S, Ravi K, Fahim M (2011) Persistence of EDHF pathway and impairment of the nitric oxide pathway after chronic mercury chloride exposure in rats: mechanisms of endothelial dysfunction. Hum Exp Toxicol 30(11):1777–1784
Branco V et al (2017) Biomarkers of mercury toxicity: past, present, and future trends. J Toxicol Environ Health B 20(3):119–154
Berglund M et al (2005) Inter-individual variations of human mercury exposure biomarkers: a cross-sectional assessment. Environ Health 4(1):1–11
Miranda GHN et al (2018) Chronic exposure to sodium fluoride triggers oxidative biochemistry misbalance in mice: effects on peripheral blood circulation. Oxidative Med Cell Longev 2018
Szász A, Barna B, Gajda Z, Galbács G, Kirsch-Volders M, Szente M (2002) Effects of continuous low-dose exposure to organic and inorganic mercury during development on epileptogenicity in rats. Neurotoxicology 23(2):197–206
Teixeira FB et al (2014) Evaluation of the effects of chronic intoxication with inorganic mercury on memory and motor control in rats. Int J Environ Res Public Health 11(9):9171–9185
Teixeira FB, Leão LKR, Bittencourt LO, Aragão WAB, Nascimento PC, Luz DA, Braga DV, Silva MCF, Oliveira KRM, Herculano AM, Maia CSF, Lima RR (2019) Neurochemical dysfunction in motor cortex and hippocampus impairs the behavioral performance of rats chronically exposed to inorganic mercury. J Trace Elem Med Biol 52:143–150
Teixeira F et al (2018) Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front Mol Neurosci 11:125
Aragão WAB et al (2018) Hippocampal dysfunction provoked by mercury chloride exposure: evaluation of cognitive impairment, oxidative stress, tissue injury and nature of cell death. Oxidative Med Cell Longev 2018
Aragao W et al (2017) Chronic exposure to inorganic mercury induces biochemical and morphological changes in the salivary glands of rats. Metallomics 9(9):1271–1278
Rufino M, Alves R, Britoetal E (2007) Determination of the total antioxidant activity in fruits by the capture of free radical DPPH. Press Release Embrapa 127:1–4
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Flohe L (1984) [10] Superoxide dismutase assays, in Methods in enzymology. Elsevier, pp 93–104
Kohn HI, Liversedge M (1944) On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J Pharmacol Exp Ther 82(3):292–300
Percário S (1994) Dosagem das LDLs modificadas através da peroxidação lipídica: correlação com o risco aterogênico. An Méd Hosp Fac Ciênc Méd Santa Casa São Paulo 13(49–52):7–9
Granger DL et al (1999) Measuring nitric oxide production in human clinical studies, in Methods in enzymology. Elsevier, pp 49–61
Nevado JB et al (2010) Mercury in the Tapajós River basin, Brazilian Amazon: a review. Environ Int 36(6):593–608
Corrêa MG et al (2020) Spinal cord neurodegeneration after inorganic mercury long-term exposure in adult rats: ultrastructural, proteomic and biochemical damages associated with reduced neuronal density. Ecotoxicol Environ Saf 191:110159
Administration., O.S.a.H.a (2012) Protecting workers from mercury exposure while crushing and recycling fluorescent bulbs. US Department of Labor, Occupational Safety and Health Administration, Washington Available from: https://www.osha.gov/Publications/mercuryexposure_fluorescentbulbs_factsheet.html. 15th Sept 2020
Martín-Doimeadios RR et al (2014) Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ Sci Pollut Res 21(12):7466–7479
Aschner M, Eberle NB, Miller K, Kimelberg HK (1990) Interactions of methylmercury with rat primary astrocyte cultures: inhibition of rubidium and glutamate uptake and induction of swelling. Brain Res 530(2):245–250
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, Feki AE (2009) Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 28(1):81–89
Bailey DM, Brugniaux JV, Filipponi T, Marley CJ, Stacey B, Soria R, Rimoldi SF, Cerny D, Rexhaj E, Pratali L, Salmòn CS, Murillo Jáuregui C, Villena M, Smirl JD, Ogoh S, Pietri S, Scherrer U, Sartori C (2019) Exaggerated systemic oxidative-inflammatory-nitrosative stress in chronic mountain sickness is associated with cognitive decline and depression. J Physiol 597(2):611–629
Baierle M et al (2015) Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med Cell Longev 2015
Lauwerys R, Bonnier C, Evrard P, Gennart JP, Bernard A (1987) Prenatal and early postnatal intoxication by inorganic mercury resulting from the maternal use of mercury containing soap. Hum Toxicol 6(3):253–256
Winship KA (1985) Toxicity of mercury and its inorganic salts. Adverse Drug React Acute Poisoning Rev 4(3):129–160
Shenker B, Rooney C, Vitale L, Shapiro IM (1992) Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. I. Suppression of T-cell activation. Immunopharmacol Immunotoxicol 14(3):539–553
Ahmad S, Mahmood R (2019) Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ Sci Pollut Res 26(6):5645–5657
Liu B, Yu H, Baiyun R, Lu J, Li S, Bing Q, Zhang X, Zhang Z (2018) Protective effects of dietary luteolin against mercuric chloride-induced lung injury in mice: involvement of AKT/Nrf2 and NF-κB pathways. Food Chem Toxicol 113:296–302
Moneim AEA (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis 30(4):935–942
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38(4):769–789
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Schalcher TR, Borges RS, Coleman MD, Batista Júnior J, Salgado CG, Vieira JL, Romão PR, Oliveira FR, Monteiro MC (2014) Clinical oxidative stress during leprosy multidrug therapy: impact of dapsone oxidation. PLoS One 9(1):e85712
Albuquerque RV, Malcher NS, Amado LL, Coleman MD, Dos Santos DC, Borges RS, Valente SA, Valente VC, Monteiro MC (2015) In vitro protective effect and antioxidant mechanism of resveratrol induced by Dapsone hydroxylamine in human cells. PLoS One 10(8):e0134768
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int 2014:1–26
Kumar A et al (2018) Biochemical and molecular targets of heavy metals and their actions, in Biomedical applications of metals. Springer, pp 297–319
Çubukçu HC, Yurtdaş M, Durak ZE, Aytaç B, Güneş HN, Çokal BG, Yoldaş TK, Durak İ (2016) Oxidative and nitrosative stress in serum of patients with Parkinson’s disease. Neurol Sci 37(11):1793–1798
Chiang P-L, Chen HL, Lu CH, Chen YS, Chou KH, Hsu TW, Chen MH, Tsai NW, Li SH, Lin WC (2018) Interaction of systemic oxidative stress and mesial temporal network degeneration in Parkinson’s disease with and without cognitive impairment. J Neuroinflammation 15(1):281
Kumar A, Pandey R, Sharma B (2020) Modulation of superoxide dismutase activity by mercury, lead, and arsenic. Biol Trace Elem Res 1–8
Zhang LJ, Li Y, Chen P, Li XM, Chen YG, Hang YY, Gong WJ (2017) A study of genotoxicity and oxidative stress induced by mercuric chloride in the marine polychaete Perinereis aibuhitensis. Environ Toxicol Pharmacol 56:361–365
Ma Y, Zheng YX, Dong XY, Zou XT (2018) Effect of mercury chloride on oxidative stress and nuclear factor erythroid 2-related factor 2 signalling molecule in liver and kidney of laying hens. J Anim Physiol Anim Nutr 102(5):1199–1209
Feng P, Wei J, Zhang Z (2011) Intervention of selenium on chronic fluorosis-induced injury of blood antioxidant capacity in rats. Biol Trace Elem Res 144(1–3):1024–1031
Dhouib IB, Annabi A, Doghri R, Rejeb I, Dallagi Y, Bdiri Y, Lasram MM, Elgaaied A, Marrakchi R, Fazaa S, Gati A (2017) Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum: biochemical, histological, and behavioral changes. Environ Sci Pollut Res 24(35):27515–27524
Milnerowicz H, Ściskalska M, Dul M (2015) Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J Trace Elem Med Biol 29:1–10
Penta KL, Fairweather D, Shirley DL, Rose NR, Silbergeld EK, Nyland JF (2015) Low-dose mercury heightens early innate response to coxsackievirus infection in female mice. Inflamm Res 64(1):31–40
Ho-Tin-Noé B, Boulaftali Y, Camerer E (2018) Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood 131(3):277–288
Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pörnbacher M, Lauber K, Krombach F, Reichel CA (2016) Platelets guide leukocytes to their sites of extravasation. PLoS Biol 14(5):e1002459
Diaz-Vivancos P, de Simone A, Kiddle G, Foyer CH (2015) Glutathione–linking cell proliferation to oxidative stress. Free Radic Biol Med 89:1154–1164
Lorscheider F, Vimy M (2000) Mercury and idiopathic dilated cardiomyopathy. J Am Coll Cardiol 35(3):819–820
Hansen JM, Zhang H, Jones DP (2006) Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med 40(1):138–145
Alissa EM, Ferns GA (2011) Heavy metal poisoning and cardiovascular disease. J Toxicol 2011:1–21
Satoh H (2000) Occupational and environmental toxicology of mercury and its compounds. Ind Health 38(2):153–164
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 supported by the Brazilian National Council for Scientific and Technological Development (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The animals in this study were supplied by the Central Animal Hospital of the Federal University of Pará under the protocol of the Ethics Committee for the Use of Animals n° 9,228,050,418.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos Chemelo, V., Bittencourt, L.O., Aragão, W.A.B. et al. Long-Term Exposure to Inorganic Mercury Leads to Oxidative Stress in Peripheral Blood of Adult Rats. Biol Trace Elem Res 199, 2992–3000 (2021). https://doi.org/10.1007/s12011-020-02411-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02411-5