Abstract
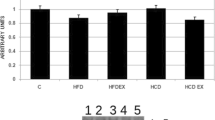
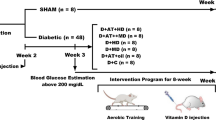
Type 2 diabetes mellitus (T2D) is a metabolic disorder caused by chronic hyperglycemia due to a deficiency in the secretion and/or action of insulin. Zinc (Zn) supplementation and strength exercise increases insulin signaling. We evaluate the effect of Zn supplementation and strength exercise on insulin resistance in the liver of rats with diet-induced T2D through the study of phosphorylation of Akt and protein tyrosine phosphatase 1B (PTP1B). Rats were fed with a high-fat diet (HFD) for 18 weeks to induce T2D and then assigned in four experimental groups: HFD, HFD-Zn (Zn), HFD-strength exercise (Ex), and HFD-Zn/strength exercise (ZnEx) and treated during 12 weeks. Serum Zn, lipid profile, transaminases, glucose, and insulin were measured. In the liver with/without insulin stimuli, total and phosphorylated Akt (pAktSer473) and PTP1B (pPTP1BSer50) were determined by western blot. Hepatic steatosis was evaluated by histological staining with red oil and intrahepatic triglyceride (IHTG) content. There were no differences in biochemical and body-related variables. The ZnEx group showed a higher level of pAkt, both with/without insulin. The ZnEx group also showed higher levels of pPTP1B with respect to HFD and Zn groups. The ZnEx group had higher levels of pPTP1B than groups treated with insulin. Liver histology showed a better integrity and less IHTG in Ex and ZnEx with respect to the HFD group. The Ex and ZnEx groups had lower IHTG with respect to the HFD group. Our results showed that Zn supplementation and strength exercise together improved insulin signaling and attenuated nonalcoholic liver disease in a T2D rat model.




Similar content being viewed by others
References
Kharroubi AT, Darwish H (2015) Diabetes mellitus: the epidemic of the century. World J Diabetes 6(6):850–867
Olokoba AB, Obateru O, Olokoba LB (2012) Type 2 diabetes mellitus: a review of current trends. Oman Med J 27(4):269–273
Fu Z, Gilbert E, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev 9(1):25–53
Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, Frier BM, Van Look LA, Johnston GI, Reynolds RM, Strachan MW (2011) Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 34(5):1139–1144
Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R, Nabavi SM (2016) Non-alcoholic fatty liver disease and diabetes. Metabolism 65(8):1096–1108
Sunny NE, Parks E, Browning JD, Burgess SC (2011) Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14:804–810
Manco M (2017) Insulin resistance and NAFLD: a dangerous liaison beyond the genetics. Children (Basel) 4(8):74
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of nonalcoholic fatty liver disease (NAFLD). Metabolism 65:1038–1044
Gurzov EN, Tran M, Fernandez-Rojo MA, Merry TL, Zhang X, Xu Y, Fukushima A, Waters MJ, Watt MJ, Andrikopoulos S, Neel BG, Tiganis T (2014) Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab 20(1):85–102
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4:82–91
Li YV (2014) Zinc and insulin in pancreatic beta-cells. Endocrine 45(2):178–189
Ranasinghe P, Pigera S, Galappatthy P, Katulanda P, Constantine GR (2015) Zinc and diabetes mellitus: understanding molecular mechanisms and clinical implications. Daru 23:44
Bellomo E, Birla-Singh K, Massarotti A, Hogstrand C, Maret W (2016) The metal face of protein tyrosine phosphatase 1B. Coord Chem Rev 327-328:70–83
Bakke J, Haj FG (2015) Protein-tyrosine phosphatase 1B substrates and metabolic regulation. Seminars in Cell & Develop Biol 37:58.65. https://doi.org/10.1016/j.semcdb.2014.09.020
Bellomo E, Masarotti A, Hogstrand C, Maret W (2014) Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics 6:1229–1239
Parsons ZD, Gates KS (2013) Thiol dependent recovery of catalytic activity from oxidized protein tyrosine phosphatases. Biochemistry 52:6412–6423
Shi K, Egawa K, Maegawa H, Nakamura T, Ugi S, Nishio Y, Kashiwagi A (2004) Protein-tyrosine phosphatase 1B associates with insulin receptor and negatively regulates insulin signaling without receptor internalization. J Biochem 136:89–96
González-Rodríguez A, Mas-Gutiérrez J, Sanz-González S, Ros M, Burks DJ, Valverde AM (2010) Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes 59:588–599
Feldhammer M, Uetani N, Miranda-Saavedra D, Tremblay ML (2013) PTP1B: a simple enzyme for a complex world. Crit Rev Biochem Mol Biol 48(5):430–445. https://doi.org/10.3109/10409238.2013.819830
Buckley DA, Cheng A, Kiely PA, Tremblay ML, O’Connor R (2002) Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol 22:1998–1910
Bhakta HK, Paudel P, Fujii H, Sato A, Park CH, Yokozawa T, Jung HA, Choi JS (2017) Oligonol promotes glucose uptake by modulating the insulin signaling pathway in insulin-resistant HepG2 cells via inhibiting protein tyrosine phosphatase 1B. Arch Pharm Res 40(11):1314–1327
Ravichandran LV, Chen H, Li Y, Quon MJ (2001) Phosphorylation of PTP1B at Ser(50) by Akt impairs its ability to dephosphorylate the insulin receptor. Mol Endocrinol 15:1768–1780
Kido K, Ato S, Yokokawa T, Makanae Y, Sato K, Fujita S (2016) Acute resistance exercise-induced IGF1 expression and subsequent GLUT4 translocation. Physiol Rep 4(16).
Pesta DH, Goncalves R, Madiraju AK, Strasser B, Sparks LM (2017) Resistance training to improve type 2 diabetes: working toward a prescription for the future. Nutr Metab (Lond) 14:24
Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG (2010) Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42(10):1843–1852
Li M, Li W, Yoon J-H, Jeon BH, Lee SK (2015) Resistance exercise training increase activation of AKT-eNOS and Ref-1 expression by FOXO-1 activation in aorta of F344 rats. J Exerc Nutr Biochem 19(3):165–171
Marinho R, Mekary R, Muñoz VR, Gomes RJ, Pauli JR, de Moura LP (2015) Regulation of hepatic TRB3/Akt interaction induced by physical exercise and its effect on the hepatic glucose production in an insulin resistance state. Diabetol Metab Syndr 7:67
Zelber-Sagi S, Bush A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, Fliss-Isakov N, Izkhakov E, Halpern Z, Santo E, Oren R, Shibolet O (2014) Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 20(15):4382–4392
Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, Shiba N, Torimura T (2017) Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 66(1):142–152
Wang Y, Wang P, Qin LQ, Davaasambuu G, Kaneko T, Xu J, Murata S, Katoh R, Sato A (2003) The development of diabetes mellitus in Wistar rats kept on a high-fat/low carbohydrate diet for long periods. Endocrine 22:85–92
Brøns C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, Jacobsen S, Nilsson E, Larsen CM, Astrup A, Quistorff B, Vaag A (2009) Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 587(Pt 10):2387–2397
Skovso S (2014) Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Invest 5(4):349–358
Tubbs E, Chanon S, Robert M, Bendridi N, Bidaux G, Chauvin M et al (2018) Disruption of mitochondria-associated endoplasmic reticulum membrane (MAM) integrity contributes to muscle insulin resistance in mice and humans. Diabetes 67(4):636–650. https://doi.org/10.2337/db17-0316
Arias EB, Zheng X, Agrawal S, Cartee GD (2019) Whole body glucoregulation and tissue-specific glucose uptake in a novel Akt substrate of 160 kDa knockout rat model. PloS One 14(4):e0216236. https://doi.org/10.1371/journal.pone.0216236
Konishi M, Sakaguchi M, Lockhart SM, Cai W, Li ME, Homan EP, Rask-Madsen C, Kahn CR (2017) Endothelial insulin receptors differentially control insulin signaling kinetics in peripheral tissues and brain of mice. PNAS USA 114(40):E8478–E8487. https://doi.org/10.1073/pnas.1710625114
Katsoulieris EN, Drossopoulou GI, Kotsopoulou ES, Vlahakos DV, Lianos EA, Tsilibary EC (2016) High glucose impairs insulin signaling in the glomerulus: an in vitro and ex vivo approach. PloS One 11(7):e0158873. https://doi.org/10.1371/journal.pone.0158873
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin Phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Barthel A, Ostrakhovitch E, Walter PL, Kampkötter A, Klotz LO (2007) Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: mechanisms and consequences. Arch Biochem Biophys 463:175–182
Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z (2009) Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol 297:569–575
Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki A, Shi K et al (2004) Protein phosphatase 2A negativaly regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3 T3-L1 adipocye. Mol Cell Biol 25:8778–8789
Xiong Y, Luo DJ, Wang XL, Qiu M, Yang Y, Yan X, Wang JZ, Ye QF, Liu R (2015) Zinc binds to and directly inhibits protein phosphatase 2A in vitro. Neurosci Bull 31:331–337
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth. autophagy and metabolism. Nat Cell Biol 13(9):1016–1023
Wieringa FT, Dijkhuizen MA, Fiorentino M, Laillou A, Berger J (2015) Determination of zinc status in humans: which indicator should we use? Nutrients 7(5):3252–3263
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: an integrative review. J Res Med Sci. 18(2):144–157
Wei CC, Luo Z, Hogstrand C, Xu YH, Wu LX, Chen GH, Pan YX, Song YF (2018) Zinc reduces hepatic lipid deposition and activates lipophagy via Zn2+/MTF-1/PPARα and Ca2+/CaMKKβ/ AMPK pathways. FASEB J Jun 28:fj201800463.
Wu Y, Lu H, Yang H, Li C, Sang Q, Liu X, Liu Y, Wang Y, Sun Z (2016) Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J Nutr Biochem 34:126–135. https://doi.org/10.1016/j.jnutbio.2016.05.008
Bellomo E, Abro A, Hogstrand C, Maret W, Domene C (2018) Role of zinc and magnesium ions in the modulation of phosphoryl transfer in protein tyrosine phosphatase 1B. J Am Chem Soc 140(12):4446–4454
Shidfar F, Faghihi A, Amiri HL, Mousavi SN (2016) Regression of nonalcoholic fatty liver disease with zinc and selenium co-supplementation after disease progression in rats. Iran J Med Sci 43(1):26–31
Himoto T, Masaki T (2018) Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients 10(1):E88
van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H (2018) The effects of physical exercise on fatty liver disease. Gene Expr 18(2):89–101
Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI (2011) Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60(9):1278–1283
Juraschek SP, Blaha M, Blumenthal RS, Brawner C, Qureshi W, Keteyian SJ, Schairer J, Ehrman JK, Al-Mallah MH (2015) Cardiorespiratory fitness and incident diabetes: the FIT (Henry Ford ExercIse Testing) project. Diabetes Care 38(6):1075–1081
Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H (2018) Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr 18:5–17
Romestaing C, Piquet MA, Bedu E, Rouleau V, Dautresme M, Hourmand-Ollivier I, Filippi C, Duchamp C, Sibille B (2007) Long term highly saturated fat diet does not induce NASH in wistar rats. Nutr Metab (Lond) 4:4. https://doi.org/10.1186/1743.7075.4.4)
Chen PJ, Cai SP, Huang C, Meng XM, Li J (2015) Protein tyrosine phosphatase 1B (PTP1B): A key regulator and therapeutic target in liver diseases. Toxicology 337:10–20
Acknowledgments
The authors sincerely acknowledge the technical support for rat diet preparation of the following undergraduate students: JF Orellana, P Meneses, C Espinoza, R Farias, M Muñoz, and A Rivas, from the School of Nutrition and Dietetics (Universidad de Chile). The authors also acknowledge the contribution of Marcelo Cano, PhD, and Alex Barham, MSc, for implementing the exercise protocol.
Funding
This work was supported by the National Commission for Research in Science and Technology (CONICYT), research project FONDECYT 1160792
Author information
Authors and Affiliations
Contributions
AV, MRu, and MAO conceptualized and designed the research. AV, KM, AE, JC, JI, and KV performed the experiments. AV, MRi, and MAO analyzed the data. AV, MRi, MRu, and MAO interpreted the results of experiments. AV, MRi, and MAO prepared the figures. AV and MA drafted the manuscript. MRu and MAO edited and revised the manuscript. MAO and MRu approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Manuel Ruz, Jorge Inostroza, Diego García, and Miguel Arredondo received payment from the research project FONDECYT 1160792 that funded this study. The rest of authors declare that they have no conflicts of interest.
Ethical Approval
Animal experiments were performed in accordance with animal protection regulations of the Faculty of Medicine, University of Chile. The protocol was approved by the Bioethics Committee, Faculty of Medicine, University of Chile.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vivero, A., Ruz, M., Rivera, M. et al. Zinc Supplementation and Strength Exercise in Rats with Type 2 Diabetes: Akt and PTP1B Phosphorylation in Nonalcoholic Fatty Liver. Biol Trace Elem Res 199, 2215–2224 (2021). https://doi.org/10.1007/s12011-020-02324-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02324-3