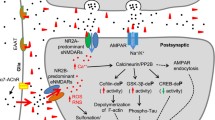
Abstract
The basal level of extracellular Zn2+ is in the range of low nanomolar (~ 10 nM) in the hippocampus. However, extracellular Zn2+ dynamics plays a key role for not only cognitive activity but also cognitive decline. Extracellular Zn2+ dynamics is modified by glutamatergic synapse excitation and the presence of amyloid-β1–42 (Aβ1–42), a causative peptide in Alzheimer’s disease (AD). When human Aβ1–42 reaches high picomolar (> 100 pM) in the extracellular compartment of the rat dentate gyrus, Zn-Aβ1–42 complexes are readily formed and taken up into dentate granule cells, followed by Aβ1–42-induced cognitive decline that is linked with Zn2+ released from intracellular Zn-Aβ1–42 complexes. Aβ1–42-induced intracellular Zn2+ toxicity is accelerated with aging because of age-related increase in extracellular Zn2+. The recent findings suggest that Aβ1–42 secreted continuously from neuron terminals causes age-related cognitive decline and neurodegeneration via intracellular Zn2+ dysregulation. On the other hand, metallothioneins (MTs), zinc-binding proteins, quickly serve for intracellular Zn2+-buffering under acute intracellular Zn2+ dysregulation. On the basis of the idea that the defense strategy against Aβ1–42-induced pathogenesis leads to preventing the AD development, this review deals with extracellular Zn2+-dependent Aβ1–42 neurotoxicity, which is accelerated with aging.




Similar content being viewed by others
References
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601
Knierim JJ (2015) The hippocampus. Curr Biol 25:R1116–R1121
Sasaki T, Leutgeb S, Leutgeb JK (2015) Spatial and memory circuits in the medial entorhinal cortex. Curr Opin Neurobiol 32:16–23
Takeda A, Tamano H (2017) Impact of synaptic Zn2+ dynamics on cognition and its decline. Int J Mol Sci 18:2411
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Muelle RN, Zeng Y, Balaji RV, Masalha R, Thompson RB, Fierke CA, Sarvey JM, Valdenebro M, Prough DS, Zornow MH (2016) Concentrations of extracellular free zinc (pZn) e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol 198:285–293
Tamano H, Nishio R, Shakushi Y, Sasaki M, Koike Y, Osawa M, Takeda A (2017) In vitro and in vivo physiology of low nanomolar concentrations of Zn2+ in artificial cerebrospinal fluid. Sci Rep 7:42897
Takeda A, Koike Y, Osawa M, Tamano H (2018) Characteristic of extracellular Zn2+ influx in the middle-aged dentate gyrus and its involvement in attenuation of LTP. Mol Neurobiol 55:2185–2195
Sensi SL, Canzoniero LMT, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW (1997) Measurement of intracellular free zinc in living cortical neurons: routes of entry. J Neurosci 15:9554–9564
Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR (2008) Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am J Phys Cell Phys 294:C726–C742
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344
Gomez-Isla T, Price JL, McKeel DW, Jr Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16:4491–4500
Scheff SW, Price DA, Schmitt FA, Mufson EJ (2006) Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384
Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet 19:R12–R20
Brouillette J, Caillierez R, Zommer N, Alves-Pires C, Benilova I, Blum D, De Strooper B, Buée L (2012) Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-β1-42 oligomers are revealed in vivo by using a novel animal model. J Neurosci 32:7852–7861
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225:1168–1170
Perrin RJ, Fagan AM, Holtzman DM (2009) Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 461:916–922
Kepp KP (2016) Alzheimer’s disease due to loss of function: a new synthesis of the available data. Prog Neurobiol 143:36–60
Welikovitch LA, Do Carmo S, Maglóczky Z, Szocsics P, Lőke J, Freund T, Cuell AC (2018) Evidence of intraneuronal Aβ accumulation preceding tau pathology in the entorhinal cortex. Acta Neuropathol 136:901–917
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 2002(298):789–791
Takeda A, Takada S, Nakamura M, Suzuki M, Tamano H, Ando M, Oku N (2011) Transient increase in Zn2+ in hippocampal CA1 pyramidal neurons causes reversible memory deficit. PLoS One 6:e28615
Suzuki M, Fujise Y, Tsuchiya Y, Tamano H, Takeda A (2015) Excess influx of Zn2+ into dentate granule cells affects object recognition memory via attenuated LTP. Neurochem Int 87:60–65
Takeda A, Nakamura M, Fujii H, Uematsu C, Minamino T, Adlard PA, Bush AI, Tamano H (2014) Amyloid β-mediated Zn2+ influx into dentate granule cells transiently induces a short-term cognitive deficit. PLoS One 9:e115923
Kim NK, Robinson HP (2011) Effects of divalent cations on slow unblock of native NMDA receptors in mouse neocortical pyramidal neurons. Eur J Neurosci 34:199–212
Jones HC, Keep RF (1988) Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol 402:579–593
Neves G, Cooke SF, Bliss TVP (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci 9:65–67
Lisman J, Yasuda R, Raghavachari S (2012) Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13:169–182
Takeda A, Suzuki M, Tempaku M, Ohashi K, Tamano H (2015) Influx of extracellular Zn2+ into the hippocampal CA1 neurons is required for cognitive performance via long-term potentiation. Neuroscience 304:209–216
Tamano H, Nishio R, Takeda A (2017) Involvement of intracellular Zn2+ signaling in LTP at perforant pathway-CA1 pyramidal cell synapse. Hippocampus 27:777–783
Tamano H, Minamino T, Fujii H, Takada S, Nakamura M, Ando M, Takeda A (2015) Blockade of intracellular Zn2+ signaling in the dentate gyrus erases recognition memory via impairment of maintained LTP. Hippocampus 25:952–962
Remondes M, Schuman EM (2004) Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431:699–703
Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S (2011) Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science 334:1415–1420
Vago DR, Kesner RP (2008) Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res 189:273–283
Sindreu CB, Varoqui H, Erickson JD, Pérez-Clausell J (2003) Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb Cortex 13:823–829
Takeda A, Tamano H, Ogawa T, Takada S, Nakamura M, Fujii H, Ando M (2014) Intracellular Zn2+ signaling in the dentate gyrus is required for object recognition memory. Hippocampus 24:1404–1412
Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313:1141–1144
Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K (2003) Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron 38:447–460
Okamoto K, Bosch M, Hayashi Y (2009) The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda) 24:357–366
Morgado-Bernal I (2011) Learning and memory consolidation: linking molecular and behavioral data. Neuroscience 176:12–19
Kägi JHR, Kojima Y (1987) Chemistry and biochemistry of metallothionein. Experientia [Suppl] 52:25–80
Palmiter RD, Findley SD, Whitmore TE, Durnam DM (1992) MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A 89:6333–6337
Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, Bush AI (1997) Metallothioneins in brain--the role in physiology and pathology. Toxicol Appl Pharmacol 142:229–242
Yanagitani S, Miyazaki H, Nakahashi Y, Kuno K, Ueno Y, Matsushita M, Naitoh Y, Taketani S, Inoue K (1999) Ischemia induces metallothionein III expression in neurons of rat brain. Life Sci 64:707–715
Penkowa M, Giralt M, Camats J, Hidalgo J (2002) Metallothionein 1+2 protect the CNS during neuroglial degeneration induced by 6-aminonicotinamide. J Comp Neurol 444:174–189
Helal GK, Aleisa AM, Helal OK, Al-Rejaie SS, Al-Yahya AA, Al-Majed AA, Al-Shabanah OA (2009) Metallothionein induction reduces caspase-3 activity and TNFalpha levels with preservation of cognitive function and intact hippocampal neurons in carmustine-treated rats. Oxidative Med Cell Longev 2:26–35
Takeda A, Tamano H, Hashimoto W, Kobuchi S, Suzuki H, Murakami T, Tempaku M, Koike Y, Adlard PA, Bush AI (2018) Novel defense by metallothionein induction against cognitive decline: from amyloid β1-42-induced excess Zn2+ to functional Zn2+ deficiency. Mol Neurobiol 55:7775–7788
Krężel A, Maret W (2017) The functions of metamorphic metallothioneins in zinc and copper metabolism. Int J Mol Sci 18
Choi DW (1998) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Swann JW, Al-Noori S, Jiang M, Lee CL (2000) Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 10:617–625
Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C (2010) Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47:264–272
Bickler PE, Warren DE, Clark JP, Gabatto P, Gregersen M, Brosnan H (2012) Anesthetic protection of neurons injured by hypothermia and rewarming: roles of intracellular Ca2+ and excitotoxicity. Anesthesiology 117:280–292
Abushik PA, Sibarov DA, Eaton MJ, Skatchkov SN, Antonov SM (2013) Kainate-induced calcium overload of cortical neurons in vitro: dependence on expression of AMPAR GluA2-subunit and down-regulation by subnanomolar ouabain. Cell Calcium 54:95–104
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10:780–791
Smith, R.M. NIST critically selected stability constants of metal complexes: version 8. NIST Scientific and Technical Databases [online], http://www.nist.gov/srd/nist46.htm (2009)
Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW (1996) The role of zinc in selective neuronal death after transient global cerebral ischemia. Science 272:1013–1016
Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV (2003) Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A 100:2906–2910
Liu S, Lau L, Wei J, Zhu D, Zou S, Sun HS, Fu Y, Liu F, Lu Y (2004) Expression of Ca(2+)-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron 43:43–55
Weiss JH (2011) Ca permeable AMPA channels in diseases of the nervous system. Front Mol Neurosci 4:42
Takeda A, Tamano H, Hisatsune M, Murakami T, Nakada H, Fujii H (2018) Maintained LTP and memory are lost by Zn2+ influx into dentate granule cells, but not Ca2+ influx. Mol Neurobiol 55:1498–1508
Tamano H, Nishio R, Morioka H, Takeda A (2019) Extracellular Zn2+ influx into nigral dopaminergic neurons plays a key role for pathogenesis of 6-hydroxydopamine-induced Parkinson’s disease in rats. Mol Neurobiol 56:435–443
Tamano H, Morioka H, Nishio R, Azusa T, Takeda A (2019) Blockade of rapid influx of extracellular Zn2+ into nigral dopaminergic neurons overcomes Paraquat-induced Parkinson’s disease in rats. Mol Neurobiol 56:4539–4548
Tamano H, Nishio R, Morioka H, Furuhata R, Komata Y, Takeda A (2019) Paraquat as an environmental risk factor in Parkinson's disease accelerates age-related degeneration via rapid influx of extracellular Zn2+ into nigral dopaminergic neurons. Mol Neurobiol 56:7789–7799
Nestor PJ, Scheltens P, Hodges JR (2004) Advances in the early detection of Alzheimer’s disease. Nat Med 10:S34–S41
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci 23:8844–8853
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48:913–922
Gu L, Tran J, Jiang L, Guo Z (2016) A new structural model of Alzheimer’s Aβ42 fibrils based on electron paramagnetic resonance data and Rosetta modeling. J Struct Biol 194:61–67
Schoonenboom NS, Mulder C, Van Kamp GJ, Mehta SP, Scheltens P, Blankenstein MA, Mehta PD (2005) Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol 58:139–142
Bush AI (2013) The metal theory of Alzheimer’s disease. J Alzheimers Dis 33:S277–S281
Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265:1464–1467
Klug GM, Losic D, Subasinghe SS, Aguilar MI, Martin LL, Small DH (2003) Beta-amyloid protein oligomers induced by metal ions and acid pH are distinct from those generated by slow spontaneous ageing at neutral pH. Eur J Biochem 270:4282–4293
Garai K, Sahoo B, Kaushalya SK, Desai R, Maiti S (2007) Zinc lowers amyloid-beta toxicity by selectively precipitating aggregation intermediates. Biochemistry 46:10655–10663
Chen WT, Liao YH, Yu HM, Cheng IH, Chen YR (2011) Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-beta stability, oligomerization, and aggregation: amyloid-beta destabilization promotes annular protofibril formation. J Biol Chem 286:9646–9656
Solomonov I, Korkotian E, Born B, Feldman Y, Bitler A, Rahimi F, Li H, Bitan G, Sagi I (2012) Zn2+−Abeta40 complexes form metastable quasi-spherical oligomers that are cytotoxic to cultured hippocampal neurons. J Biol Chem 287:20555–20564
Lee MC, Yu WC, Shih YH, Chen CY, Guo ZH, Huang SJ, Chan JCC, Chen YR (2018) Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer's disease. Sci Rep 8:4772
Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G (2000) High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Boil Chem 277:32046–32053
McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, DeLucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T (2005) Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron 47:191–199
Selkoe DJ (2008) Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res 192:106–113
Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO (2010) Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat Struct Mol Biol 17:561–567
Masuda Y, Uemura S, Ohashi R, Nakanishi A, Takegoshi K, Shimizu T, Shirasawa T, Irie K (2009) Identification of physiological and toxic conformations in Abeta42 aggregates. Chembiochem 10:287–295
Xiao Y, Ma B, McElheny D, Parthasarathy S, Long F, Hoshi M, Nussinov R, Ishii Y (2015) Aβ(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat Struct Mol Biol 22:499–505
Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI (2000) Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem 75:1219–1233
Huang X, Atwood CS, Moir RD, Hartshorn MA, Vonsattel JP, Tanzi RE, Bush AI (1997) Zinc-induced Alzheimer’s Abeta1-40 aggregation is mediated by conformational factors. J Biol Chem 272:26464–26470
Syme CD, Viles JH (2006) Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Abeta) of Alzheimer’s disease. Biochim Biophys Acta 1764:246–256
Danielsson J, Pierattelli R, Banci L, Graslund A (2007) High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid beta-peptide. FEBS J 274:46–59
Watt NT, Whitehouse IJ, Hooper NM (2011) The role of zinc in Alzheimer’s disease. Int J Alzheimers Dis 2011(971021)
Tõugu V, Karafin A, Palumaa P (2008) Binding of zinc (II) and copper (II) to the full-length Alzheimer’s amyloid-beta peptide. J Neurochem 104:1249–1259
Takeda A, Tamano H, Tempaku M, Sasaki M, Uematsu C, Sato S, Kanazawa H, Datki ZL, Adlard PA, Bush AI (2017) Extracellular Zn2+ is essential for amyloid β1-42-induced cognitive decline in the normal brain and its rescue. J Neurosci 37:7253–7262
Tamano H, Suzuki H, Kobuchi S, Adlard PA, Bush AI, Takeda A (2019) Difference in ability for extracellular Zn2+ influx between human and rat amyloid β1-42 and its significance. NeuroToxicology 72:1–5
Wang T, Wang CY, Shan ZY, Teng WP, Wang ZY (2012) Clioquinol reduces zinc accumulation in neuritic plaques and inhibits the amyloidogenic pathway in AβPP/PS1 transgenic mouse brain. J Alzheimers Dis 29:549–559
Matlack KE, Tardiff DF, Narayan P, Hamamichi S, Caldwell KA, Caldwell GA, Lindquist S (2014) Clioquinol promotes the degradation of metal-dependent amyloid-β (Aβ) oligomers to restore endocytosis and ameliorate Aβ toxicity. Proc Natl Acad Sci U S A 111:4013–4018
Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW (2008) Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebocontrolled trial. Lancet Neurol 7:779–786
Adlard PA, Bush AI (2018) Metals and Alzheimer’s disease: how far have we come in the clinic? J Alzheimers Dis 62:1369–1379
Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, Terasaki T, Hashimoto T, Iwatsubo T (2009) Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci 29:11393–11398
Tamano H, Takiguchi M, Shimaya R, Adlard PA, Bush AI, Takeda A (2019) Extracellular Zn2+-independently attenuated LTP by human amyloid β1-40 and rat amyloid β1-42. Biochem Bioph Res Co 514:888–892
Tamano H, Oneta N, Shioya A, Adlard PA, Bush AI, Takeda A (2019) In vivo synaptic activity-independent co-uptakes of amyloid β1-42 and Zn2+ into dentate granule cells in the normal brain. Sci Rep 9:6498
Arispe N, Rojas E, Pollard HB (1993) Giant multilevel cation channels formed by Alzheimer disease amyloid ß protein [Aß-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A 90:10573–10577
Shirwany NA, Payette D, Xie J, Guo Q (2007) The amyloid beta ion channel hypothesis of Alzheimer’s disease. Neuropsychiatr Dis Treat 3:597–612
Tamano H, Takiguchi M, Tanaka Y, Murakami T, Adlard PA, Bush AI, Takeda A (2019) Preferential neurodegeneration in the dentate gyrus by amyloid β1-42-induced intracellular Zn2+ dysregulation and its defense strategy. Mol Neurobiol:1–14. https://doi.org/10.1007/s12035-019-01853-w
Foster TC (2007) Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6:319–325
Kumar A, Bodhinathan K, Foster TC (2009) Susceptibility to calcium dysregulation during brain aging. Front Aging Neurosci 1:2
Toescu EC, Vreugdenhil M (2010) Calcium and normal brain ageing. Cell Calcium 47:158–164
Berridge MJ (2013) Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 7:2–13
Takeda A, Tamano H, Murakami T, Nakada H, Minamino T, Koike Y (2018) Weakened intracellular Zn2+-buffering in the aged dentate gyrus and its involvement in erasure of maintained LTP. Mol Neurobiol 55:3856–3865
Colvin RA, Holmes WR, Fontaine CP, Maret W (2010) Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2(306–317)
Krężel A, Maret W (2006) Zinc buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 11:1049–1062
Krężel A, Hao Q, Maret W (2007) The zinc/thiolate redox biochemistry of metallothionein and the control of zinc ion fluctuations in cell signaling. Arch Biochem Biophys 463:188–200
Vasák M, Kägi JH (1983) Spectroscopic properties of metallothionein. In: Sigel H (ed) metal ions in biological systems, vol 15. Marcel Dekker, New York, pp 213–273
Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 7:337–347
Bousleiman J, Pinsky A, Ki S, Su A, Morozova I, Kalachikov S, Wiqas A, Silver R, Sever M, Austin RN Function of metallothionein-3 in neuronal cells: do metal ions alter expression levels of MT3? Int J Mol Sci 18:1133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, Y., Takiguchi, M., Tamano, H. et al. Extracellular Zn2+-Dependent Amyloid-β1–42 Neurotoxicity in Alzheimer’s Disease Pathogenesis. Biol Trace Elem Res 199, 53–61 (2021). https://doi.org/10.1007/s12011-020-02131-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02131-w