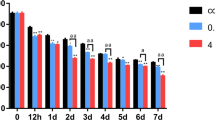
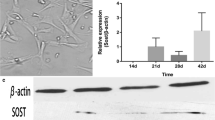
Abstract
Excessive intake of fluoride inhibits bone growth in both humans and animals. It is unknown whether fluoride acts directly on the growth plate to inhibit longitudinal bone growth, and its mechanism of action has not been elucidated. In this study, we used an organ culture system and SW1353 cells to evaluate the effects of fluoride on endochondral ossification. Neonatal rat metatarsal bones were dissected and cultured with or without fluoride for 7 days. The total length and width of the metatarsal rudiments and the length of the calcification zone were measured. Chondrocyte proliferation, differentiation, and apoptosis were analyzed by immunohistochemistry and TUNEL assay in sectioned bones. The apoptosis was detected by flow cytometry, and the expression of apoptosis-related proteins Bax, Bcl-2, and Caspase-3 were detected by western blotting in SW1353 cells. Linear measurements demonstrated that fluoride induced a biphasic effect on longitudinal bone growth in organ culture, with a significant growth inhibition at a high concentration (10−4 M) and a stimulatory action at low concentration (10−6 M) of fluoride. Histomorphometrical analysis of growth plate from fluoride-exposed metatarsal rudiments showed a significant reduction in the height of the proliferative and hypertrophic chondrocyte zones. Analysis of the Col2α1 and Col10α1 expression by immunohistochemistry revealed fluoride-suppressed metatarsal growth plate chondrocyte proliferation and differentiation. In addition, fluoride increased the number of apoptotic chondrocytes in the metatarsal growth plate. Western blotting showed an up-regulated expression of Caspase-3 and Bax and down-regulated expression of anti-apoptotic protein Bcl-2 after treatment with 5 × 10−4 M fluoride in SW1353 cells. Our findings indicated that fluoride inhibited longitudinal bone growth by acting directly at the growth plate in cultured neonatal rat metatarsal bones. Such growth inhibition was mediated by suppressing proliferation and differentiation, increasing apoptosis of resting chondrocytes and causing premature cell senescence in the growth plate.







Similar content being viewed by others
Abbreviations
- Col2α1 :
-
Type II collagen
- Col10a1 :
-
Type X collagen
- RZ:
-
Resting zone
- PZ:
-
Proliferative zone
- HZ:
-
Hypertrophic zone
References
Jiang N, Guo F, Sun B, Zhang X, Xu H (2019) Different effects of fluoride exposure on the three major bone cell types. Biol Trace Elem Res:1–8. https://doi.org/10.1007/s12011-019-01684-9
Yesildag A, Heybeli N, Candir O, Oyar O, Baykal B, Mumcu E, Gulsoy U (2004) Effects of fluoride on growth plate cartilage in rats radiological and histopathological findings. Fluoride. 37(3):21–30. https://doi.org/10.1016/j.chemosphere.2018.04.144
Guo X, Xu P, Kang L, Cao H, Du X, Mark Hvd, Mark Kvd (2002) Effects of excessive fluoride ingestion in rats on differential expression of collagen types and chondrocyte differentiation in cartilage. Fluoride. 35 (2):90–103. https://doi.org/10.1016/0029-554X(73)90656-3
Linhares D, Camarinho R, Garcia PV, Rodrigues ADS (2018) Mus musculus bone fluoride concentration as a useful biomarker for risk assessment of skeletal fluorosis in volcanic areas. Chemosphere. 205(8):540–544. https://doi.org/10.1016/j.chemosphere.2018.04.144
Kebede A, Retta N, Abuye C, Whiting SJ, Kassaw M, Zeru T, Tessema M, Kjellevold M (2016) Dietary fluoride intake and associated skeletal and dental fluorosis in school age children in rural Ethiopian rift valley. Int J Environ Res Public Health 13(8):756–766. https://doi.org/10.3390/ijerph13080756
Bonyadi RE, Musumeci G, Pichler K, Heidary M, Szychlinska MA, Castrogiovanni P, Marth E, Böhm C, Srinivasaiah S, Krönke G, Weinberg A, Schäfer U (2017) Runx2 mediated induction of novel targets ST2 and Runx3 leads to cooperative regulation of hypertrophic differentiation in ATDC5 chondrocytes. Sci Rep 7(1):17947. https://doi.org/10.1038/s41598-017-18044-z
Pichler K1, Musumeci G, Vielgut I, Martinelli E, Sadoghi P, Loreto C, Weinberg AM (2013) Towards a better understanding of bone bridge formation in the growth plate-an immunohistochemical approach. Connect Tissue Res 54(6):408–415. https://doi.org/10.3109/03008207.2013.828715
Srinivasaiah S, Musumeci G, Mohan T, Castrogiovanni P, Absenger-Novak M, Zefferer U, Mostofi S, Bonyadi RE, Grün NG, Weinberg AM, Schäfer U (2019) A 300 μm organotypic bone slice culture model for temporal investigation of endochondral osteogenesis. Tissue Eng Part C Methods 25(4):197–212. https://doi.org/10.1089/ten.TEC.2018.0368
Pichler K, Kraus T, Martinelli E, Sadoghi P, Musumeci G, Uggowitzer PJ, Weinberg AM (2014) Cellular reactions to biodegradable magnesium alloys on human growth plate chondrocytes and osteoblasts. Int Orthop 38(4):881–889. https://doi.org/10.1007/s00264-013-2163-3
Zhong M, Carney DH, Jo H, Boyan BD, Schwartz Z (2011) Inorganic phosphate induces mammalian growth plate chondrocyte apoptosis in mitochondrial pathway involving nitric oxide and JNK MAP kinase. Calcif Tissue Int 88(2):96–108. https://doi.org/10.1007/s00223-010-9433-5
Zaman F, Menendez-Benito V, Eriksson E, Chagin AS, Takigawa M, Fadeel B, Dantuma NP, Chrysis D, Sävendahl L (2007) Proteasome inhibition up-regulates p53 and apoptosis-inducing factor in chondrocytes causing severe growth retardation in mice. Cancer res. 67 (20)78-86. https://doi.org/10.1158/0008-5472.CAN-06-3982
Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM (2013) Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci 14(8):67–84. https://doi.org/10.3390/ijms140815767
Zaman F, Chrysis D, Huntjens K, Fadeel B, Sävendahl L (2012) Ablation of the pro-apoptotic protein Bax protects mice from glucocorticoid-induced bone growth impairment. PLoS One 7(3):168–180. https://doi.org/10.1371/journal.pone.0033168
Tsang KY, Tsang SW, Chan D, Cheah KS (2014) The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C Embryo Today 102(1):52–73. https://doi.org/10.1002/bdrc.21060
Liu Q, Liu H, Yu X, Wang Y, Yang C, Xu H (2016) Analysis of the role of insulin signaling in bone turnover induced by fluoride. Biol Trace Elem Res 171(2):380–390. https://doi.org/10.1007/s12011-015-0555-5
Chagin AS, Karimian E, Sundstrom K, Eriksson E, Savendahl L (2010) Catch-up growth after dexamethasone withdrawal occurs in cultured postnatal rat metatarsal bones. J Endocrinol 204(1):21–29. https://doi.org/10.1677/joe-09-0307
Gebauer M, Saas J, Sohler F, Haag J, Soder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T (2005) Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1β. Osteoarthr Cartil 13(8):697–708. https://doi.org/10.1016/j.joca.2005.04.004
Coxam V, Miller MA, Bowman MB, Miller SC (1996) Ontogenesis of IGF regulation of longitudinal bone growth in rat metatarsal rudiments cultured in serum-free medium. Arch Physiol Biochem 104(2):173–179. https://doi.org/10.1076/apab.104.2.173.12893
Cho SM, Lee SH, Lee D, Lee JH, Chang GT, Kim H, Lee JY (2017) The Korean herbal formulation Yukmijihwangtang stimulates longitudinal bone growth in animal models. BMC Complement Altern Med 17(1):239–246. https://doi.org/10.1186/s12906-017-1651-1
Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ (1991) Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res 9(3):348–359. https://doi.org/10.1002/jor.1100090306
Webster SV, Farquharson C, Jefferies D, Kwan AP (2003) Expression of type X collagen, Indian hedgehog and parathyroid hormone related-protein in normal and tibial dyschondroplastic chick growth plates. Avian Pathol 32(1):69–80. https://doi.org/10.1080/030794502/000070741
Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J (2002) The role of the resting zone in growth plate chondrogenesis. Endocrinology. 143(5):1851–1857. https://doi.org/10.1210/endo.143.5.8776
Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, Nagasawa T, Kronenberg HM, Ono N (2018) Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 563(7730):254–258. https://doi.org/10.1038/s41586-018-0662-5
Ono N, Ono W, Nagasawa T, Kronenberg HM (2014) A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol 16(12):1157–1167. https://doi.org/10.1038/ncb3067
Hinton RJ, Jing Y, Jing J, Feng JQ (2017) Roles of chondrocytes in endochondral bone formation and fracture repair. J Dent Res 96(1):23–30. https://doi.org/10.1177/0022034516668321
Kronenberg HM (2003) Developmental regulation of the growth plate. Nature. 423(6937):332–336. https://doi.org/10.1038/nature01657
Aurich M, Hofmann GO, Gras F, Rolauffs B (2018) Human osteochondritis dissecans fragment-derived chondrocyte characteristics ex vivo, after monolayer expansion-induced de-differentiation, and after re-differentiation in alginate bead culture. BMC Musculoskelet Disord 19(1):168–178. https://doi.org/10.1186/s12891-018-2079-6
Epasinghe DJ, Kwan S, Chu D, Lei MM, Burrow MF, Yiu CKY (2017) Synergistic effects of proanthocyanidin, tri-calcium phosphate and fluoride on artificial root caries and dentine collagen. Mater Sci Eng C Mater Biol Appl 73(4):293–299. https://doi.org/10.1016/j.msec.2016.11.078
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45(6):487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Zaman F, Zhao Y, Celvin B, Mehta HH, Wan J, Chrysis D, Ohlsson C, Fadeel B, Cohen P, Sävendahl L (2019) Humanin is a novel regulator of hedgehog signaling and prevents glucocorticoid-induced bone growth impairment. FASEB J 33(4):4962–4974. https://doi.org/10.1096/fj.201801741R
Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW, Lazebnik Y, Flavell RA (2000) Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med 6(11):1241–1247. https://doi.org/10.1038/81343
Van Opdenbosch N, Lamkanfi M (2019) Caspases in cell death, inflammation, and disease. Immunity. 50(6):1352–1364. https://doi.org/10.1016/j.immuni.2019.05.020
Ramirez MLG, Salvesen GS (2018) A primer on caspase mechanisms. Semin Cell Dev Biol 82(10):79–85. https://doi.org/10.1016/j.semcdb.2018.01.002
Chrysis D, Ritzen EM, Savendahl L (2003) Growth retardation induced by dexamethasone is associated with increased apoptosis of the growth plate chondrocytes. J Endocrinol 176(3):331–337. https://doi.org/10.1677/joe.0.1760331
Thompson EM, Matsiko A, Farrell E, Kelly DJ, O'Brien FJ (2015) Recapitulating endochondral ossification: a promising route to in vivo bone regeneration. J Tissue Eng Regen Med 9(8):889–902. https://doi.org/10.1002/term.1918
Eriksson E, Zaman F, Chrysis D, Wehtje H, Heino TJ, Sävendahl L (2012) Bortezomib is cytotoxic to the human growth plate and permanently impairs bone growth in young mice. PLoS One 7(11):e50523. https://doi.org/10.1371/journal.pone.0050523
Fernandez-Vojvodich P, Zaman F, Sävendahl L (2013) Interleukin-6 acts locally on the growth plate to impair bone growth. Ann Rheum Dis 72(10):e24. https://doi.org/10.1136/annrheumdis-2013-204112
Funding
This work was supported by a grant from the National Natural Science Foundation of China (NSFC) (No. 81573100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed according to the norms of the local ethics committee at China Medical University in accordance with the national guidelines for animal use and care.
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, R., Liu, S., Qiao, T. et al. Fluoride Inhibits Longitudinal Bone Growth by Acting Directly at the Growth Plate in Cultured Neonatal Rat Metatarsal Bones. Biol Trace Elem Res 197, 522–532 (2020). https://doi.org/10.1007/s12011-019-01997-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01997-9