Abstract
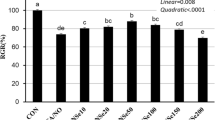
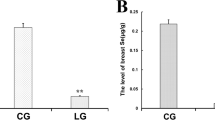
The objective of this study was to determine the effects of selenium (Se) on antioxidative function and the synthesis of milk protein in bovine mammary epithelial cells (BMECs). Two experiments were conducted using a single-factor completely randomized design study. In part I, BMECs were randomly divided into seven groups: control (without Se) and six Se treatments (10, 20, 50, 100, 150, and 200 nmol/L). In part II, based on the results of part I, we used lipopolysaccharide (LPS) as the induced stress source to analyze the protective effect of Se on LPS-induced oxidative damage and the influence on milk protein synthesis of BMECs. BMECs were randomly divided into eight groups: control (without Se and LPS), LPS treatment (only LPS), and six Se treatments with LPS (LS10 to LS200). Treatment of BMECs with Se was found to significantly improve cell proliferation and antioxidant function. LPS could induce oxidative damage which significantly inhibited cell proliferation and antioxidant function in BMECs. Se had a protective effect on the oxidative damage of BMECs induced by LPS. Additionally, our results indicated that LPS damage downregulated the gene expression of milk protein synthesis. Se effectively relieved the inhibition due to LPS-induced oxidative damage on the synthesis of milk protein, and Se concentrations of 50 to 200 nmol/L showed the best effect. In conclusion, Se at concentrations of 50 to 100 nmol/L is better for antioxidant function but had no effect on milk protein synthesis in healthy BMECs. Se ameliorated the damage caused by LPS-induced by improving levels of antioxidant markers and upregulating milk protein synthesis and the expression of genes associated with milk protein in BMECs.
Similar content being viewed by others
References
Ametaj BN, Emmanuel DGV, Zebeli Q et al (2009) Feeding high proportions of barley grain in a total mixed ration perturbs diurnal patterns of plasma metabolites in lactating dairy cows[J]. J Dairy Sci 92(3):1084–1091. https://doi.org/10.3168/jds.2008-1465
Dong GZ, Liu SM, Wu YX, Lei CL, Zhou J, Zhang S (2011) Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: Impacts on immunity an metabolism. Acta Vet Scand 53:48. https://doi.org/10.1186/1751-0147-53-48
Sen Z (2012) Lipopolysaccharide as an abnormal metabolite in the rumen: effects on plasma metabolites and hormones and mechanisms in dairy cows[J]. Chin J Anim Nutri 48(4):24. https://doi.org/10.3176/lu.2012.4.01
Shi H, Guo Y, Liu Y et al (2016) The in vitro effect of lipopolysaccharide on proliferation, inflammatory factors and antioxidant enzyme activity in bovine mammary epithelial cells[J]. Anim Nutr 2(02):41–46 http://doi.org/CNKI:SUN:ANNU.0.2016-02-007
Lixin L, Ye L, Li Z et al (2015) Cytotoxicity of LPS and effects on milk protein synthesis in dairy cow mammary epithelial cells[J]. J Northeast Agricult Univ 46(6):61–66
Gong J, Ni L, Wang D et al (2014) Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows[J]. Livest Sci 2014(170):84–90. https://doi.org/10.1016/j.livsci.2014.10.003
Guo Y, Boqi Z, Sumei Y, Binlin S, Guo X (2017) Protective effects of selenium on bovine mammary epithelial cells oxidative damage by lipopolysaccharide [J]. Chin J Anim Nutr 29(9):3375–3384
Sheng R, Yan SM, Qi LZ et al (2015) (2015). Effect of the ratios of unsaturated fatty acids on the expressions of genes related to fat and protein in the bovine mammary epithelial cells[J]. In Vitro Cell Dev Biol Anim 51(4):381–389. https://doi.org/10.1007/s11626-014-9847-x
Robinson TL, Sutherland IA, Sutherland J (2007) Validation of candidate bovine reference genes for use with real-time PCR[J]. Vet Immunol Immunopathol 115(1-2):165. https://doi.org/10.1016/j.vetimm.2006.09.012
Zhang W, Zhang R, Wang T et al (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture [J]. Inflammation 37(2):478. https://doi.org/10.1007/s10753-013-9761-5
Wei Z, Yao M, Li Y et al (2014) Dietary selenium deficiency exacerbates lipopolysaccharide-induced inflammatory response in mouse mastitis models[J]. Inflammation 37(6):1925–1931. https://doi.org/10.1007/s10753-014-9925-y
Wei Z, Yao M, Li Y et al (2015) Retraction note to: Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by selenium in bovine mammary epithelial cells in primary culture[J]. Inflammation 38(4):1662–1662. https://doi.org/10.1007/s10753-015-0142-0
Bionaz M, Loor JJ (2011) Gene networks driving bovine mammary protein synthesis during the lactation cycle [J]. Bioinform Biol Insights 2011(5):83–98. https://doi.org/10.4137/BBI.S7003
Bernard L, Leroux C, Chilliard Y (2008) Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland [J]. Adv Exp Med Biol 2008(606):67–108. https://doi.org/10.1007/978-0-387-74087-4_2
Yang X, Yin L, Li T et al (2014) Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPα and PPARγ[J]. Int J Clin Exp Med 7(12):4906–4914
Liu L, Lin Y, Liu L, Bian Y, Zhang L, Gao X, Li Q (2015) 14-3-3γ regulates lipopolysaccharide-induced inflammatory responses and lactation in dairy cow mammary epithelial cells by inhibiting NF-κB and MAPKs and up-regulating mTOR signaling [J]. Int J Mol Sci 16(7):16622–16641. https://doi.org/10.3390/ijms160716622
Silanikove N, Rauch-Cohen A, Shapiro F et al (2012) Lipopolysaccharide challenge of the mammary gland in cows induces nitrosative stress that impairs milk oxidative stability [J]. Animal 2012(6):1451–1459. https://doi.org/10.1017/S1751731112000201
Schmitz S, Pfaffl MW, Meyer HH et al (2004) Short-term changes of mRNA expression of various inflammatory factors and milk proteins in mammary tissue during LPS-induced mastitis [J]. Domest Anim Endocrinol 26(2):111–126. https://doi.org/10.1016/j.domaniend.2003.09.003
Bionaz M, Loor JJ (2008) Gene networks driving bovine milk fat synthesis during the lactation cycle [J]. BMC Genomics 9(1):366. https://doi.org/10.1186/1471-2164-9-366
Blanchard PG, Festuccia WT, Houde VP, St-Pierre P, Brûlé S, Turcotte V, Côté M, Bellmann K, Marette A, Deshaies Y (2012) Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion [J]. J Lipid Res 53(6):1117–1125. https://doi.org/10.1194/jlr.M021485
Gao X, Zhang Z, Li Y et al (2016) Selenium deficiency deteriorate the inflammation of S. aureus infection via regulating NF-κB and PPAR-γ in mammary gland of mice [J]. Biol Trace Elem Res 172(1):140–147. https://doi.org/10.1007/s12011-015-0563-5
Yu X, Shao XG, Sun H et al (2008) Activation of cerebral peroxisome proliferator-activated receptors gamma exerts neuroprotection by inhibiting oxidative stress following pilocarpine-induced status epilepticus [J]. Brain Res 1200(1):146–158. https://doi.org/10.1016/j.brainres.2008.01.047
Salman S, Kholparisini A, Schafft H et al (2009) The role of dietary selenium in bovine mammary gland health and immune function [J]. Anim Health Res Rev 10(1):21–34. https://doi.org/10.1017/S1466252308001588
Acknowledgments
The authors are grateful to Yongmei Guo, Xiaoyu Guo and Yanli Zhao for their assistance during the experiments.
Funding
This study was supported by the National Natural Science Foundation of China (Project No. 31560650).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, B., Guo, Y., Yan, S. et al. The protective effect of selenium on the lipopolysaccharide-induced oxidative stress and depressed gene expression related to milk protein synthesis in bovine mammary epithelial cells. Biol Trace Elem Res 197, 141–148 (2020). https://doi.org/10.1007/s12011-019-01961-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01961-7