Abstract
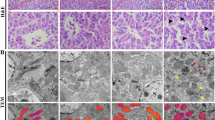
Manganese (Mn) pollution is an important environmental problem because of the potential toxicity to human and animal health. However, the effects of Mn on energy metabolism and autophagy are not clear. Consequently, we examined the effects of excessive and chronic exposure to Mn on liver function, oxidative stress, respiratory chain complex activity, and autophagy in chicken liver. Our results indicated that the accumulation of Mn in the liver and levels of AST and ALT in the serum of the Mn-exposed group were significantly higher (P < 0.05) than those in the control group at 90 days; the activities of GSH-Px, SOD, CAT, Na+-K+-ATPase, Mg2+-ATPase, Ca2+-ATPase, and respiratory chain complexes (I, II, III) in the Mn-exposed group were significantly decreased (P < 0.05) compared to the control group. However, the MDA content, NO content, iNOS activity, mRNA and protein levels of iNOS, and autophagy-related genes in the Mn-exposed group were significantly increased (P < 0.05) compared to the control group. In contrast, the mRNA level and protein expression of mTOR were significantly decreased (P < 0.05) compared to the control group. Furthermore, the characteristic autophagic vacuolar organelles were observed in the Mn-exposed group. These results suggested that excess Mn exposure can cause a disorder of energy metabolism by mitochondrial injury and induce oxidative stress and autophagy, which eventually lead to liver damage.




Similar content being viewed by others
References
Leach RM, Harris ED (1997) Manganese. In: O’Dell BL, Sunde RA (eds) Handbook of nutritionally essential elements. Marcel Dekker, New York, pp 335–355
Wei T, Quareshy M, Zhang YZ, Scanlan DJ, Chen Y (2018) Manganese is essential for PlcP metallophosphoesterase activity involved in lipid remodelling in abundant marine heterotrophic bacteria. Appl Environ Microbiol 84
Wang L, Yu H, Yang G, Zhang Y, Wang W, Su T, Ma W, Yang F, Chen L, He L, Ma Y, Zhang Y (2015) Correlation between bone mineral density and serum trace element contents of elderly males in Beijing urban area. Int J Clin Exp Med 8:19250–19257
Cailliatte R, Schikora A, Briat JF, Mari S, Curie C (2010) High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22:904–917
Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203
Aguirre JD, Culotta VC (2012) Battles with iron: manganese in oxidative stress protection. J Biol Chem 287:13541–13548
Saleh AA, Eltantawy MS, Gawish EM, Younis HH, Amber KA, Abd El-Moneim AEE, Ebeid TA (2019) Impact of dietary organic mineral supplementation on reproductive performance, egg quality characteristics, lipid oxidation, ovarian follicular development, and immune response in laying hens under high ambient temperature. Biol Trace Elem Res:1–9. https://doi.org/10.1007/s12011-019-01861-w
Xu XR, Li RB, Wang WH, Gu JD (2005) Decolorization of dyes and textile waste water by potassium permanganate. Chemom Intell Lab Syst 59:893–898
Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG (2011) Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol 256:227–240
Marks B, Peters A, McGough D (2017) Aquatic environmental risk assessment of manganese processing industries. Neurotoxicology 58:187–193
Oladipo OO, Ayo JO, Ambali SF, Mohammed B, Aluwong T (2017) Dyslipdemia induced by chronic low dose co-exposure to lead, cadmium and manganese in rats: the role of oxidative stress. Environ Toxicol Pharmacol 53:199–205
Mackenzie Ross S (2016) Delayed cognitive and psychiatric symptoms following methyl iodide and manganese poisoning: potential for misdiagnosis. Cortex 74:427–439
Guo Z, Zhang Z, Wang Q, Zhang J, Wu S (2018) Manganese chloride induces histone acetylation changes in neuronal cells: its role in manganese-induced damage. Neurotoxicology 65:255–263
Tong Y, Zhai Q, Lu W, Tian F, Chen W (2017) New insights in integrated response mechanism of Lactobacillus plantarum under excessive manganese stress. Food Res Int 102:323–332
Xing H, Li S, Wang Z, Gao X, Xu S, Wang X (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383
Wang Y, Zhao H, Shao Y, Liu J, Li J, Luo L, Xing M (2018) Copper (II) and/or arsenite-induced oxidative stress cascades apoptosis and autophagy in the skeletal muscles of chicken. Chemosphere 206:597–605
Jiao X, Yang K, An Y, Teng X, Teng X (2017) Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ Sci Pollut Res Int 24:7555–7564
Wang X, An Y, Jiao W, Zhang Z, Han H, Gu X, Teng X (2018) Selenium protects against lead-induced apoptosis via endoplasmic reticulum stress in chicken kidneys. Biol Trace Elem Res 182:354–363
Jin X, Xu Z, Zhao X, Chen M, Xu S (2017) The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 180:259–266
Chaudhary S, Iram S, Raisuddin S, Parvez S (2015) Manganese pre-treatment attenuates cadmium induced hepatotoxicity in Swiss albino mice. J Trace Elem Biol Med 29:284–288
Miguel CC, Juliana NY, Betzabet QV (2015) Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci 9:1–22
Wang Y, Wang K, Huang H, Gu X, Teng X (2017) Alleviative effect of selenium on inflammatory damage caused by lead via inhibiting inflammatory factors and heat shock proteins in chicken testes. Environ Sci Pollut Res Int 24:13405–13413
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Singh D, Kumar V, Singh C (2017) IFN-γ regulates xanthine oxidase-mediated iNOS-independent oxidative stress in maneb- and paraquat-treated rat polymorphonuclear leukocytes. Mol Cell Biochem 427:133–143
Weidinger A, Kozlov AV (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules 5:472–484
Jin X, Jia T, Liu R, Xu S (2018) The antagonistic effect of selenium on cadmium-induced apoptosis via PAR-γ/PI3K/Akt pathway in chicken pancreas. J Hazard Mater 357:355–362
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26:232–236
Riley LG, Cowley MJ, Gayevskiy V, Roscioli T, Thorburn DR, Prelog K, Bahlo M, Sue CM, Balasubramaniam S, Christodoulou J (2017) A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J Inherit Metab Dis 40:261–269
Cao B, Camden AJ, Parnell LA, Mysorekar IU (2017) Autophagy regulation of physiological and pathological processes in the female reproductive tract. Am J Reprod Immunol 77:1–7
Chen M, Li X, Fan R, Yang J, Jin X, Hamid S, Xu S (2017) Cadmium induces BNIP3-dependent autophagy in chicken spleen by modulating miR-33-AMPK axis. Chemosphere 194:396–402
Wang S, Xu Z, Yin H, Min Y, Li S (2018) Alleviation mechanisms of selenium on cadmium-spiked in chicken ovarian tissue: perspectives from autophagy and energy metabolism. Biol Trace Elem Res 186:521–528
Wang D, Zhang J, Jiang W, Cao Z, Zhao F, Cai T, Aschner M, Luo W (2017) The role of NLRP3-CASP1 in inflammasome mediated neuroinflammation and autophagy dysfunction in manganese induced, hippocampal-dependent impairment of learning and memory ability. Autophagy 13:914–927
Guo Z, Zhang Z, Wang Q, Zhang J, Wang L, Zhang Q, Li H, Wu S (2018) Manganese chloride induces histone acetylation changes in neuronal cells: Its role in manganese-induced damage. Neurotoxicology 65:255–263
Sun B, Xing M (2016) Evaluated the twenty six elements in the pectoral muscle of As-treated chicken by inductively coupled plasma mass spectrometry. Biol Trace Elem Res 169:359–364
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dyebinding. Anal Biochem 72:248–254
Xing H, Wang Z, Gao X, Chen D, Wang L, Li S, Xu S (2015) Atrazine and chlorpyrifos exposure induces liver autophagic response in common carp. Ecotoxicol Environ Saf 113:52–58
Li X, Xing M, Chen M, Zhao J, Fan R, Zhao X, Cao C, Yang J, Zhang Z, Xu S (2017) Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol Environ Saf 139:447–453
Ogbomida ET, Nakayama SMM, Bortey-Sam N, Oroszlany B, Tongo I, Enuneku AA, Ozekeke O, Ainerua MO, Fasipe IP, Ezemonye LI, Mizukawa H, Ikenaka Y, Ishizuka M (2018) Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria. Ecotoxicol Environ Saf 113:98–108
Abdel-Wahhab MA, Aly SE (2005) Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol 25:218–223
Salem R, El-Habashi N, Fadl SE, Sakr OA, Elbialy ZI (2018) Effect of probiotic supplement on aflatoxicosis and gene expression in the liver of broiler chicken. Ecotoxicol Environ Saf 60:118–127
Bahar E, Lee GH, Bhattarai KR, Lee HY, Kim HK, Handigund M, Choi MK, Han SY, Chae HJ, Yoon H (2017) Protective role of quercetin against manganese-induced injury in the liver, kidney, and lung; and hematological parameters in acute and subchronic rat models. Drug Des Devel Ther 11:2605–2619
Mohammed AT, Ebraheim LLM, Metwally MMM (2018) Ebselen can protect male reproductive organs and male fertility from manganese toxicity: structural and Bioanalytical approach in a rat model. Biomed Pharmacother 102:739–748
Khrer JP (1993) Free radicals as mediator of tissue injury and disease. Crit Rev Toxicol 23:21–48
Packialakshmi B, Zhou X (2018) Experimental autoimmune encephalomyelitis (EAE) up-regulates the mitochondrial activity and manganese superoxide dismutase (MnSOD) in the mouse renal cortex. PLoS One 13:e0196277
Rifaioglu MM, Motor S, Davarci I, Tuzcu K, Sefil F, Davarci M, Nacar A (2014) Protective effect of ebselen on experimental testicular torsion and detorsion injury. Andrologia 46:1134–1140
Uyeturk U, Terzi EH, Gucuk A, Kemahli E, Ozturk H, Tosun M (2013) Prevention of torsion-induced testicular injury by Rhodiola rosea. Urology 82:254.e1–254.e6
Lemly AD (2014) Teratogenic effects and monetary cost of selenium poisoning of fish in Lake Sutton, North Carolina. Ecotoxicol Environ Saf 104:160–167
Song XB, Liu G, Liu F, Yan ZG, Wang ZY, Liu ZP, Wang L (2017) Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis 8:e2863
Wang XY, Yang H, Wang MG, Yang DB, Wang ZY, Wang L (2017) Trehalose protects against cadmium-induced cytotoxicity in primary rat proximal tubular cells via inhibiting apoptosis and restoring autophagic flux. Cell Death Dis 8:e3099
Mortezavi A, Salemi S, Kranzbühler B, Gross O, Sulser T, Simon HU, Eberli D (2018) Inhibition of autophagy significantly increases the antitumor effect of Abiraterone in prostate cancer. World J Urol 37:351–358
Zhou Q, Fu X, Wang X, Wu Q, Lu Y, Shi J, Klaunig JE, Zhou S (2018) Autophagy plays a protective role in Mn-induced toxicity in PC12 cells. Toxicology 394:45–53
Funding
This work is supported by the Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (TDJH201804) and Innovation Foundation of Heilongjiang Bayi Agricultural University (XA2016-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were approved by the Animal Experiments Committee of the Heilongjiang Bayi Agricultural University (registration protocol 11/2016).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, J., Wang, F., Wang, L. et al. Manganese Chloride Exposure Causes Disorder of Energy Metabolism and Induces Oxidative Stress and Autophagy in Chicken Liver. Biol Trace Elem Res 197, 254–261 (2020). https://doi.org/10.1007/s12011-019-01960-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01960-8