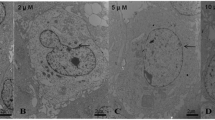
Abstract
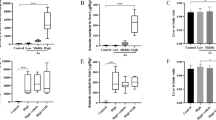
Arsenic trioxide (ATO), a trivalent arsenic compound, is known to disrupt redox homeostasis. Methionine sulfoxide reductases (Msrs), a group of antioxidant proteins, convert methionine sulfoxide back to methionine in living organisms exposed to oxidative stress. The objective of this study was to determine the effects of ATO on oxidative stress and the expressions of Msrs in mouse liver. Sixty male mice were randomly divided into six equal groups: one control group and five groups that received ATO treatment (0.3, 1, 3, 6, and 9 mg/kg, respectively). After a 4-week treatment, livers specimens were collected and assayed for malonyldialdehyde (MDA) content, superoxide dismutase (SOD) activity, total antioxidant capacity (T-AOC), and glutathione peroxidase (GSH-Px) activity. In addition, the mRNA expressions of SOD-1 and HO-1 and the mRNA and protein expressions of Msrs were also determined. Results showed that the T-AOC activity, SOD activity, and SOD-1 mRNA expression were significantly decreased (P < 0.01), while the GSH-Px level, MDA content, and HO-1 mRNA expression were significantly increased in mice treated with ATO compared with control. Levels of MsrB2 mRNA and MsrA protein were significantly increased by ATO treatment, except in the highest dose group. There were no significant changes in MsrB3 mRNA level. ATO, at 1 or 3 mg/kg, increased MsrB1 expression. Modifications in MsrA protein level were consistent with changes in mRNA levels. Collectively, our results suggest that ATO induced oxidative stress and then led to the variations in Msrs activity in mouse liver.




Similar content being viewed by others
References
Del Razo LM, Quintanilla-Vega B, Brambila-Colombres E, Calderón-Aranda ES, Manno M, Albores A (2001) Stress proteins induced by arsenic. Toxicol Appl Pharmacol 177(2):132–148
Guo Y, Zhao P, Guo G, Hu Z, Tian L, Zhang K, Sun Y, Zhang X, Zhang W, Xing M (2015) Effects of arsenic trioxide exposure on heat shock protein response in the immune organs of chickens. Biol Trace Elem Res 169(1):134–141
Cantor KP (2001) Invited commentary: arsenic and cancer of the urinary tract. Am J Epidemiol 153(5):422–423
Frumkin H, Thun MJ (2010) Arsenic. Cancer J Clin 51(4):254–262
Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC (2001) Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ Health Perspect 109(10):1011–1017
Kessel M, Liu SX, Xu A, Santella R, Hei TK (2002) Arsenic induces oxidative DNA damage in mammalian cells. Mol Cell Biochem 234-235(1):301–308
Enterline PE, Day R, Marsh GM (1995) Cancers related to exposure to arsenic at a copper smelter. Occup Environ Med 52(1):28–32
Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25(3–4):207–218
Gladyshev VN (2014) Symposium 6: trace elements S6-1 – selenium and methionine sulfoxide reduction. Free Radic Biol Med 75:S8–S9
Kim H, Gladyshev V (2007) Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J 407(3):321–329
Lee BC, Dikiy A, Kim HY, Gladyshev VN (2009) Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta 1790(11):1471–1477
Weissbach H, Resnick L, Brot N (2005) Methionine sulfoxide reductases: history and cellular role in protecting against oxidative damage. Biochim Biophys Acta 1703(2):203–212
Kaya A, Lee BC, Gladyshev VN (2015) Regulation of protein function by reversible methionine oxidation and the role of selenoprotein MsrB1. Antioxid Redox Signal 23(10):814–822
Moskovitz (2005) Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 1703(2):213–219
Ugarte N, Petropoulos I, Friguet B (2010) Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal 13(4):539–549
Alarifi S, Ali D, Alkahtani S, Siddiqui MA, Ali BA (2013) Arsenic trioxide-mediated oxidative stress and genotoxicity in human hepatocellular carcinoma cells. Onco Targets Ther 6:75–84
Kumar S, Yedjou CG, Tchounwou PB (2014) Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res 33:42
Sener U, Uygur R, Aktas C, Uygur E, Erboga M, Balkas G, Caglar V, Kumral B, Gurel A, Erdogan H (2016) Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Ren Fail 38(1):117–123
Zhang Y, Wei Z, Liu W, Wang J, He X, Huang H, Zhang J, Yang Z (2016) Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget 8(3):3773
Liu J, Zhao H, Wang Y, Shao Y, Li J, Xing M (2018) Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ Sci Pollut Res Int (Suppl 1):1–12
Kitchin KT, Ahmad S (2003) Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett 137(1):3–13
Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhaynrich C, Shimojo NJEHP (2002) Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. 110 (4):331–336
Shi H, Hudson LG, Ding W, Wang S, Cooper KL, Liu S, Chen Y, Shi X, Liu KJ (2004) Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol 17(7):871–878
Roy P, Sengupta A, Joardar N, Bhattacharyya A, Saha NC, Misra AK, Babu SPS (2019) Influence of autophagy, apoptosis and their interplay in filaricidal activity of C-cinnamoyl glycosides. Parasitology:1–38
Liu J, Zhao H, Wang Y, Shao Y, Zong H, Zeng X, Xing M (2019) Arsenic trioxide and/or copper sulfate induced apoptosis and autophagy associated with oxidative stress and perturbation of mitochondrial dynamics in the thymus of Gallus gallus. Chemosphere 219:227–235
Yang F, Liao J, Pei R, Yu W, Han Q, Li Y, Guo J, Hu L, Pan J, Tang Z (2018) Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 204:36–43
Ahmad S, Kitchin KT, Cullen WR (2000) Arsenic species that cause release of Iron from ferritin and generation of activated oxygen. Arch Biochem Biophys 382(2):195–202
Gebel T (2000) Confounding variables in the environmental toxicology of arsenic. Toxicology 144(1):155–162
Kleiner D, Bersenyi A, Febel H, Hegedus V, Matis E, Sardi E (2013) Transmethylation and the redox homeostasis. Orv Hetil 154(30):1180–1187
Luo S, Levine RL (2009) Methionine in proteins defends against oxidative stress. FASEB J 23(2):464–472
Moskovitz J, Flescher E, Berlett BS, Azare J, Poston JM, Stadtman ER (1998) Overexpression of peptide-methionine sulfoxide reductase in Saccharomyces cerevisiae and human T cells provides them with high resistance to oxidative stress. Proc Natl Acad Sci U S A 95(24):14071–14075
Skaar EP, Tobiason DM, Quick J, Judd RC, Weissbach H, Etienne F, Brot N, Seifert HS (2002) The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A 99(15):10108–10113
Sreekumar PG, Kannan R, Yaung J, Spee CK, Ryan SJ, Hinton DR (2005) Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem Biophys Res Commun 334(1):245–253
Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, Reddy VN, Hejtmancik JF (2004) Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc Natl Acad Sci U S A 101(26):9654–9659
Kwak GH, Kim JR, Kim HY (2009) Expression, subcellular localization, and antioxidant role of mammalian methionine sulfoxide reductases in Saccharomyces cerevisiae. BMB Rep 42(2):113–118
Cabreiro F, Picot CR, Perichon M, Castel J, Friguet B, Petropoulos I (2008) Overexpression of mitochondrial methionine sulfoxide reductase B2 protects leukemia cells from oxidative stress-induced cell death and protein damage. J Biol Chem 283(24):16673–16681
Boschi-Muller S, Branlant G (2014) Methionine sulfoxide reductase: chemistry, substrate binding, recycling process and oxidase activity. Bioorg Chem 57:222–230
Acknowledgments
All authors thank Shuai Zhang, Xinyan Ma, Feiyang Ma, and Yuyin Lin for their help in the animal experiments in the clinical veterinary medicine laboratory at the College of Veterinary Medicine, South China Agricultural University.
Funding
This work was supported by the National Natural Science Foundation of China (31402264, 31572585), Guangzhou Planned Program in Science and Technology (No. 201803020003), and the National Key R&D Program of China (grant numbers 2016YFD0501205, 2017YFD0502200).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal procedures were approved by the Institutional Animal Care and Use Committee of the South China Agricultural University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, G., Wan, F., Yan, H. et al. Methionine Sulfoxide Reductases Are Related to Arsenic Trioxide-Induced Oxidative Stress in Mouse Liver. Biol Trace Elem Res 195, 535–543 (2020). https://doi.org/10.1007/s12011-019-01881-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01881-6