Abstract
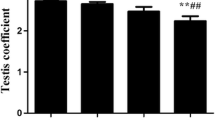
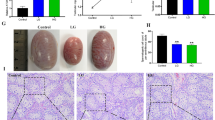
Chronic copper exposure impaired spermatogenesis in adult male mice. The aim of this study was to determine whether chronic copper exposure can induce apoptosis of testicular cell and hypospermatogenesis via disturbing testosterone synthesis in adult male mice. In the present study, sixty CD-1 male mice were randomly divided into four groups, and were continuously administered for 8 weeks by oral gavage with copper sulfate at a dose of 0, 25, 100, and 150 mg/kg/day, respectively. We determined the content of serum and testicular copper, testicular coefficient, testicular histopathology, sperm count and motility, the mRNA and protein levels of Caspase-3, Bax, and Bcl-2, Leydig cell count, testosterone content, testosterone synthetase, and testosterone synthesis–related genes. The results showed that the copper levels in serum increased in a dose-dependent manner, and the copper levels in testes were significantly related to serum copper levels. Male mice given copper sulfate 100 and 150 dosage groups showed significant decreased in sperm motility and sperm number as well as increased in testes damage, and there was no significant change in testicular coefficient in the four groups. The mRNA levels of Bcl-2 decreased and Caspase-3 increased in 150 dosage group, and Bax increased in two higher dosage groups. Meanwhile, Caspase-3 and Bax proteins increased in 150 dosage group, and Bcl-2 protein decreased in three copper treatment groups. Nevertheless, there were no differences on the levels of testosterone content and testosterone synthetase of 3β-HSD, 17β-HSD, 17α-Hyd, and 20α-Hyd, mRNA levels of Cyp11a1, Cyp17a1, and Star, and quantity of Leydig cells in four groups. Overall, these data showed that chronic copper exposure led to copper residues in the testes, and the doses of 100 and 150 mg/kg/day copper sulfate may induce hypospermatogenesis by increasing apoptosis without affecting testosterone secretion.




Similar content being viewed by others
References
Gaetke LM, Chow-Johnson HS, Chow CK (2014) Copper: toxicological relevance and mechanisms. Arch Toxicol 88(11):1929–1938. https://doi.org/10.1007/s00204-014-1355-y
Macpherson IS, Murphy MEP (2007) Type-2 copper-containing enzymes. Cell Mol Life Sci Cmls 64(22):2887–2899
Scheiber I, Dringen R, Mercer JF (2013) Copper: effects of deficiency and overload. Met Ions Life Sci 13:359–387. https://doi.org/10.1007/978-94-007-7500-8_11
Wu H, Yang F, Li H, Li Q, Zhang F, Ba Y, Cui L, Sun L, Lv T, Wang N, Zhu J (2019) Heavy metal pollution and health risk assessment of agricultural soil near a smelter in an industrial city in China. International journal of environmental health research:1–13. https://doi.org/10.1080/09603123.2019.1584666
Sodango TH, Li X, Sha J, Bao Z (2018) Review of the spatial distribution, source and extent of heavy metal pollution of soil in China: impacts and mitigation approaches. J Health Pollut 8(17):53–70. https://doi.org/10.5696/2156-9614-8.17.53
WHO (2017) Guidelines for drinking-water quality, 4th edition, incorporating the 1st addendum. World Health Organization:341
Cholewinska E, Ognik K, Fotschki B, Zdunczyk Z, Juskiewicz J (2018) Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS One 13(5):e0197083. https://doi.org/10.1371/journal.pone.0197083
Mugford C, Gibbs JL, Boylstein R (2017) Elemental properties of copper slag and measured airborne exposures at a copper slag processing facility. J Occup Environ Hyg 14(8):D120–D129. https://doi.org/10.1080/15459624.2017.1316388
Hostynek JJ (2003) Factors determining percutaneous metal absorption. Food Chem Toxicol 41(3):327–345
Agata MS, Kruczek M, Joanna K (2014) Effect of copper exposure on reproductive ability in the bank vole (Myodes glareolus). Ecotoxicology (London, England) 23(8):1546–1554
Roychoudhury S, Nath S, Massanyi P, Stawarz R, Kacaniova M, Kolesarova A (2016) Copper-induced changes in reproductive functions: in vivo and in vitro effects. Physiol Res 65(1):11–22
Ghobrial IM, Witzig TE, Adjei AA (2005) Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 55(3):178–194
Ribeiro DA, Cardoso CM, Yujra VQ, DEBV M, Aguiar O Jr, Pisani LP, CTF O (2017) Fluoride induces apoptosis in mammalian cells: in vitro and in vivo studies. Anticancer Res 37(9):4767–4777. https://doi.org/10.21873/anticanres.11883
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Yang J, Hu S, Rao M, Hu L, Lei H, Wu Y, Wang Y, Ke D, Xia W, Zhu CH (2017) Copper nanoparticle-induced ovarian injury, follicular atresia, apoptosis, and gene expression alterations in female rats. Int J Nanomedicine 12:5959–5971. https://doi.org/10.2147/ijn.S139215
Guo H, Li K, Wang W, Wang C, Shen Y (2017) Effects of copper on hemocyte apoptosis, ROS production, and gene expression in white shrimp Litopenaeus vannamei. Biol Trace Elem Res 179(2):318–326. https://doi.org/10.1007/s12011-017-0974-6
Zhao D, Zhang X, Li X, Ru S, Wang Y, Yin J, Liu D (2019) Oxidative damage induced by copper in testis of the red swamp crayfish Procambarus clarkii and its underlying mechanisms. Aqua Toxicol (Amsterdam, Netherlands) 207:120–131. https://doi.org/10.1016/j.aquatox.2018.12.006
Kowal M, Lenartowicz M, Pecio A, Golas A, Blaszkiewicz T, Styrna J (2010) Copper metabolism disorders affect testes structure and gamete quality in male mice. Syst Biol Reprod Med 56(6):431–444. https://doi.org/10.3109/19396361003734624
Walker WH (2011) Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 1(2):116–120
Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y (2015) Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev 24(4):459–470. https://doi.org/10.1089/scd.2014.0370
Han A, Zou L, Gan X, Li Y, Liu F, Chang X, Zhang X, Tian M, Li S, Su L, Sun Y (2018) ROS generation and MAPKs activation contribute to the Ni-induced testosterone synthesis disturbance in rat Leydig cells. Toxicol Lett 290:36–45. https://doi.org/10.1016/j.toxlet.2018.03.016
Zeng Q, Zhou B, Feng W, Wang YX, Liu AL, Yue J, Li YF, Lu WQ (2013) Associations of urinary metal concentrations and circulating testosterone in Chinese men. Reprod Toxicol (Elmsford, NY) 41:109–114. https://doi.org/10.1016/j.reprotox.2013.06.062
Erboga M, Kanter M, Aktas C, Bozdemir Donmez Y, Fidanol Erboga Z, Aktas E, Gurel A (2016) Anti-apoptotic and anti-oxidant effects of caffeic acid phenethyl ester on cadmium-induced testicular toxicity in rats. Biol Trace Elem Res 171(1):176–184. https://doi.org/10.1007/s12011-015-0509-y
Chang CS, Choi JB, Kim HJ, Park SB (2011) Correlation between serum testosterone level and concentrations of copper and zinc in hair tissue. Biol Trace Elem Res 144(1–3):264–271. https://doi.org/10.1007/s12011-011-9085-y
Chattopadhyay A, Sarkar M, Biswas NM (2005) Dose-dependent effect of copper chloride on male reproductive function in immature rats. Kathmandu Univ Med J (KUMJ) 3(4):392–400
Ramaswamy S, Weinbauer GF (2015) Endocrine control of spermatogenesis: role of FSH and LH/ testosterone. Spermatogenesis 4(2):e996025–e996025. https://doi.org/10.1080/21565562.2014.996025
Epstein O, Spisni R, Parbhoo S, Woods B, Dormandy T (1982) The effect of oral copper loading and portasystemic shunting on the distribution of copper in the liver, brain, kidney, and cornea of the rat. Am J Clin Nutr 35(3):551–555. https://doi.org/10.1093/ajcn/35.3.551
Hebert CD, Elwell MR, Travlos GS, Fitz CJ, Bucher JR (1993) Subchronic toxicity of cupric sulfate administered in drinking water and feed to rats and mice. Fundam Appl Toxicol 21(4):461–475
Sakhaee E, Emadi L, Abshenas J, Kheirandish R, Azari O, Amiri E (2012) Evaluation of epididymal sperm quality following experimentally induced copper poisoning in male rats. J Andrologia 44(s1):110–116
Huang Q, Luo L, Alamdar A, Zhang J, Liu L, Tian M, Eqani SA, Shen H (2016) Integrated proteomics and metabolomics analysis of rat testis: mechanism of arsenic-induced male reproductive toxicity. Sci Rep 6:32518. https://doi.org/10.1038/srep32518
Kang Z, Qiao N, Tan Z, Tang Z, Li Y (2017) Expression patterns and changes of the LCN2 gene in the testes of induced cryptorchidism and busulfan-treated mice. Syst Biol Reprod Med 63(6):364–369. https://doi.org/10.1080/19396368.2017.1355416
Miska-Schramm A, Kruczek M, Kapusta J (2014) Effect of copper exposure on reproductive ability in the bank vole (Myodes glareolus). Ecotoxicology (London, England) 23(8):1546–1554. https://doi.org/10.1007/s10646-014-1295-6
Ruwanpura SM, McLachlan RI, Meachem SJ (2010) Hormonal regulation of male germ cell development. J Endocrinol 205(2):117–131. https://doi.org/10.1677/joe-10-0025
Doguer C, Ha J-H, Collins JF (2018) Intersection of Iron and copper metabolism in the mammalian intestine and liver. Compr Physiol 8(4):1433–1461. https://doi.org/10.1002/cphy.c170045
Ha J-H, Doguer C, Collins JF (2017) Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am J Physiol Gastrointest Liver Physiol 313(4):G535–G360. https://doi.org/10.1152/ajpgi.00169.2017
Kuo YM, Zhou B, Cosco D, Gitschier J (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A 98(12):6836–6841. https://doi.org/10.1073/pnas.111057298
Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR (2006) Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr 136(1):21–26. https://doi.org/10.1093/jn/136.1.21
Lee J, Prohaska JR, Thiele DJ (2001) Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A 98(12):6842–6847. https://doi.org/10.1073/pnas.111058698
Zhang ZW, Zhi G, Qiao N, Kang ZL, Chen ZL, Hu LM, Yang ZM, Li Y (2016) Copper-induced spermatozoa head malformation is related to oxidative damage to testes in CD-1 mice. Biol Trace Elem Res 173(2):427–432. https://doi.org/10.1007/s12011-016-0675-6
Mandil R, Rahal A, Prakash A, Garg SK, Gangwar NK, Swain DK (2016) Ameliorative potential of alpha-tocopherol against flubendiamide and copper-induced testicular-insult in Wistar rats. Chem Biol Interact 260:91–101. https://doi.org/10.1016/j.cbi.2016.11.004
Musacco-Sebio R, Ferrarotti N, Saporito-Magrina C, Semprine J, Fuda J, Torti H, Boveris A, Repetto MG (2014) Oxidative damage to rat brain in iron and copper overloads. Metallomics 6(8):1410–1416. https://doi.org/10.1039/c3mt00378g
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87(7):1157–1180. https://doi.org/10.1007/s00204-013-1034-4
Lin J, Zhao H-S, Qin L, Li X-N, Zhang C, Xia J, Li J-L (2018) Atrazine triggers mitochondrial dysfunction and oxidative stress in quail (Coturnix C. coturnix) cerebrum via activating xenobiotic-sensing nuclear receptors and modulating cytochrome P450 systems. J Agric Food Chem 66(25):6402–6413. https://doi.org/10.1021/acs.jafc.8b01413
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2(9):647–656. https://doi.org/10.1038/nrc883
Zeng Q, Yi H, Huang L, An Q, Wang H (2018) Reduced testosterone and Ddx3y expression caused by long-term exposure to arsenic and its effect on spermatogenesis in mice. Environ Toxicol Pharmacol 63:84–91. https://doi.org/10.1016/j.etap.2018.08.012
Payne AH, Hales DB (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25(6):947–970. https://doi.org/10.1210/er.2003-0030
Zang ZJ, Fang YQ, Ji SY, Gao Y, Zhu YQ, Xia TT, Jiang MH, Zhang YN (2017) Formaldehyde inhibits sexual behavior and expression of steroidogenic enzymes in the testes of mice. J Sex Med 14(11):1297–1306. https://doi.org/10.1016/j.jsxm.2017.09.001
Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM (2003) Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci 73(2):431–441. https://doi.org/10.1093/toxsci/kfg087
Khushboo M, Murthy MK, Devi MS, Sanjeev S, Ibrahim KS, Kumar NS, Roy VK, Gurusubramanian G (2018) Testicular toxicity and sperm quality following copper exposure in Wistar albino rats: ameliorative potentials of L-carnitine. Environ Sci Pollut Res Int 25(2):1837–1862. https://doi.org/10.1007/s11356-017-0624-8
Funding
This research was supported by the National Key R & D Program of China [grant nos. 2016YFD0501205 and 2017YFD0502200], the National Science Foundation for Young Scientists of China [grant no. 31502131], and the Youth Teacher Award Foundation of South China Agricultural University [Provided by Guangdong Wens Dahuanong Biotechnology Co., Ltd.; grant no. 5500-A17003].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Animal procedures were approved by the Institutional Animal Care and Use Committee of the South China Agricultural University
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Kang, Z., Qiao, N. et al. Chronic Copper Exposure Induces Hypospermatogenesis in Mice by Increasing Apoptosis Without Affecting Testosterone Secretion. Biol Trace Elem Res 195, 472–480 (2020). https://doi.org/10.1007/s12011-019-01852-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01852-x