Abstract
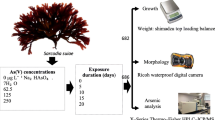
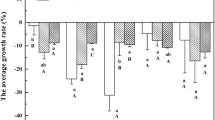
Arsenic is a noted dangerous metalloid found in many organisms, including humans, that accumulate via food consumption of aquatic products such as macroalgae, particularly where they are a major component of the human diet. The mechanism of accumulation of inorganic arsenic (iAs) as the most toxic form of arsenic (As) was investigated under three different light intensities (LI) (30, 55, and 80 μmol photons m−2 s−1) at varied arsenite (As (III)) concentrations (conc) (0, 125, 250, and 500 μg L−1) using Sarcodia suiae, a red marine macroalga. The depigmentation of the algal fronds from deep red to slightly pinkish-orange and solid green has been confirmed as a form of developmental acclimation, and the direct toxic effects of conc and LI were manifested by the degree of severity of this symptom. Two-way ANOVA showed a significant difference in iAs accumulation which depended upon conc and LI. Stepwise regression analysis showed LI as the second most important variable after conc in all treatments. S. suiae did not appear to intracellularly transform As (III) to arsenate (As (V)); hence, As (III) oxidation seemed to be a preliminary biotransformation as reflected by the dominance and increased accumulation of toxic As (III) by the alga. These findings may render it a prospective environmentally friendly candidate for reducing toxic As hazard risk and as a biological component in the treatment of wastewater. Moreover, these results also suggest that a more concerted effort is required in developing protocols for public health concerns regarding food safety and quality regulations in seafood and products sourced from macroalgae including S. suiae.




Similar content being viewed by others
References
O’Connor TP (2004) The sediment quality guideline, ERL, is not a chemical concentration at the threshold of sediment toxicity. Marine Poll Bull 49:383–385
Agency for Toxic Substances and Disease Registry (2017) Priority list of hazardous substances. Retrieved from: http://www.atsdr.cdc.gov/spl/index.html
Francesconi KA (2010) Arsenic species in seafood: origin and human health implications. Pure Appl Chem 82:373–381
Souri Z, Karimi N, Sandalio LM (2017) Arsenic hyperaccumulation strategies: an overview. Front Cell Dev Biol 5:67
World Health Organization (2011) Arsenic in drinking-water
Thundiyil JG, Yuan Y, Smith AH, Steinmaus C (2007) Seasonal variation of arsenic concentration in wells in Nevada. Environ Res 104:367–373
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Clemens S, Ma JF (2016) Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu Rev Plant Biol 67:89–512
National Research Council (1999) Arsenic in drinking water. The National Academies Press; National Research Council, Washington, DC
Ramasamy S, Lee JS (2015) Arsenic risk assessment. Handbook of arsenic toxicology edited by S. J. S. Flora 95–111
Conti ME, Iacobucci M, Cecchetti G (2007) A biomonitoring study: trace metals in seagrass, algae and molluscs in a marine reference ecosystem (southern Tyrrhenian Sea). Intl J Environ Pollut 29(1–3):308–332
Gubelit Y, Polyak Y, Dembska G, Pazikowska–Sapota G, Zegarowski L, Kochura D, Krivorotov D, Podgornaya E, Burova O, Maazouzi C (2016) Nutrient and metal pollution of the eastern gulf of Finland coastline sediments, macroalgae, microbiota. Sci Total Environ 550:806–819
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2013) Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ Sci Pollut R 20(5):3011–3023
Duncan EG, Maher WA, Foster SD (2015) Contribution of arsenic species in unicellular algae to the cycling of arsenic in marine ecosystems. Environ Sci Technol 49:33–35
Yin XX, Wang LH, Bai R, Huang H, Sun GX (2012) Accumulation and transformation of arsenic in the blue-green alga Synechocysis sp. PCC6803. Water Air Soil Pollut 223:1183–1190
Markley CT, Herbert BE (2010) Modelling phosphate influence on arsenate reduction kinetics by a freshwater cyanobacterium. Environ Model Assess 15:361–368
Campbell KM, Nordstrom DK (2014) Arsenic speciation and sorption in natural environments. Rev Mineral Geochem 79(1):185–216
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sust Energ Rev 50:431–444
Chen B, Zou D, Zhu M, Yang Y (2016) Effects of CO2 levels and light intensities on growth and amino acid contents in red seaweed Gracilaria lemaneiformis. Aquac Res:1–8
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17:61–66
Yadav G, Srivastava PK, Singh VP, Prasad S (2014) Light intensity alters the extent of arsenic toxicity in Helianthus annuus L. seedlings. Biol Trace Elem Res 158:410–421
Duncan E, Foster S, Maher W (2010) Uptake and metabolism of arsenate, methylarsonate and arsenobetaine by axenic cultures of the phytoplankton Dunaliella tertiolecta. Bot Mar 53:377–386
Yamaoka Y, Takimura O, Fuse O (1988) Environmental factors relating to arsenic accumulation by Dunaliella sp. App Organomet Chem 2:359–6364
Lin SM, Tseng LC, Ang PO Jr, Bolton J, Liu LC (2018) Long–term study on seasonal changes in floristic composition and structure of marine macroalgal communities along the coast of Northern Taiwan, southern East China Sea. Mar Biol 165:83
Abtahi M, Mesdaghinia A, Saeedi R, Nazmara S (2013) Biosorption of As (III) and As(V) from aqueous solutions by brown macroalga Colpomenia sinuosa biomass: kinetic and equilibrium studies. Desalin Water Treat 51(16–18):3224–3232
Tabaraki R, Heidarizadi E (2018) Simultaneous biosorption of arsenic (III) and arsenic (V): application of multiple response optimizations. Ecotox Environ Safe 166:35–41
Geiszinger A, Goessler W, Pedersen SN, Francesconi KA (2001) Arsenic biotransformation by the brown macroalga Fucus serratus. Environ Toxicol Chem 20:2255–2262
Rodriguez-Prieeto C, De Clerck O, Kitayama T, Lin SM (2017) Systematic revision of the widespread species Sarcodia ceylanica (Sarcodiaceae, Rhodophyta) in the Indo-Pacific oceans, including S. suiae sp. nov. Phycologia 56:63–76
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Publishing Corporation, New York, pp 29–60
Lee PY, Wu TM (2018) Studies on the toxicity of inorganic arsenic to Sarcodia suieae. Thesis, National Pingtung University of Science and Technology
Lee MC, Libatique MJH, Yeh SY (2018) The effect of environmental factors on total arsenic accumulation in Sarcodia suiae, Rhodophyta. Bull Environ Contam Toxicol 102(3):385–390
Choi H, Park SK, Kim DS, Kim M (2011) Determination of 6 arsenic species present in seaweed by solvent extraction, clean-up, and LC–ICP/MS. Food Sci Biotechnol 20:39–44
Ackley KL, B’Hymer C, Sutton KL, Caruso JA (1999) Speciation of arsenic in fish tissue using microwave-assisted extraction followed by HPLC-ICP-MS. J Anal At Spectrom 14:845–850
Brisbin JA, B’Hymer C, Caruso JA (2002) A gradient anion exchange chromatographic method for the speciation of arsenic in lobster tissue extracts. Talanta 58:133–145
Chapman PM, Wang FY (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22
Sizova OI, Kochetkov VV, Validov SZ, Boronin AM, Kosterin PV, Lyubun YV (2002) Arsenic-contaminated soils: genetically modified Pseudomonas spp. and their arsenic-phytoremediation potential. J Soils Sediment 2:19–23
Wang Z, Luo Z, Yan C (2013) Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ Sci Pollut R 20:7286–7295
Zeraatkar AZ, Ahmadzadeh H, Talebi AF, Moheimani NR, McHenry MP (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manag:1–15
Vialet-Chabrand S, Matthews JS, Simkin AJ, Raines CA, Lawson T (2017) Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol 173:2163–2179
Pandey S, Rai R, Rai LC (2012) Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J Proteome 75:921–937
Miyake C, Amako K, Shiraishi N, Sugimoto T (2009) Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system–relationship among the fraction of open PSII centers, non-photochemical quenching of chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol 50:730–743
Gu J, Zhou Z, Li Z, Chen Y, Wang Z, Zhang H, Yang J (2017) Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Front Plant Sci 8:1082
Pospíšil P (2016) Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci 7:1950
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. BBA–Bioenergetics 1143:113–134
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Miyao M (1994) Involvement of active oxygen species in degradation of the D1 protein under strong illumination in isolated supercomplexes of photosystem II. Biochem 33:9722–9730
Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition – an historical perspective. Photosynth Res 76:343–370
Nishiyama Y, Allakverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757:742–749
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:339–359
Horiguchi T (1988) Mechanism of manganese toxicity and tolerance of plants VII. Effect of light intensity on manganese– induced chlorosis. J Plant Nutr 11:235–245
Wissemeier AH, Horst WJ (1992) Effects of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata). Plant Soil 143:299–309
Bottino N, Newman R, Cox E, Stockton R, Hoban M, Zingaro R, Irgolic KN (1978) The effects of arsenate and arsenite on the growth and morphology of the marine unicellular algae Tetraselmis chui (Chlorophyta) and Hymenomonas carterae (Chrysophyta). J Exp Mar Biol Ecol 33:153–168
Adams ML, Zhao FJ, McGrath SP, Nicholson FA, Chambers BJ (2004) Predicting cadmium concentrations in wheat and barley grain using soil properties. J Environ Qual 33:532–541
Wang Z, Luo Z, Yan C (2013) Accumulation, transformation, and release of inorganic arsenic by the freshwater cyanobacterium Microcystis aeruginosa. Environ Sci Pollut R 20:7286–7295
Qin J, Lehr CR, Yuan C, Le XC, McDermott TR, Rosen BP (2009) Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc Natl Acad Sci U S A 106:5213–5217
Srivastava S, Penna S, D’Souza SF (2012) Mechanisms of arsenic tolerance ad detoxification in plants and their application in transgenic technology: a critical appraisal. Int J Phytoremediation 14:506–517
Bahar MM, Megharaj M, Naidu R (2013) Toxicity, transformation and accumulation of inorganic arsenic species in a microalga Scenedesmus sp. isolated from soil. J Appl Phycol 25:913–917
Jahan K, Mosto P, Mattson C, Frey E, Derchak L (2006) Microbial removal of arsenic. Water Air Soil Pollut 6:71–82
Papadopoulos A, Prochaska C, Papadopoulos F, Gantidis N, Metaxa E (2007) Determination and evaluation of cadmium, copper, nickel, and zinc in agricultural soils in Western Macedonia, Greece. Environ Manag 40:719–726
Sharma VK, Sohn M (2009) Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environ Int 35:743–759
Acknowledgements
The authors are thankful to the Director of the Traceability Certification and Inspection Center at National Taiwan Ocean University (Dr. Nan, Fan–Hua) for providing necessary laboratory facilities to carry out the work that made this paper possible.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Libatique, M.J.H., Lee, M. & Yeh, H. Effect of Light Intensity on the Mechanism of Inorganic Arsenic Accumulation and Patterns in the Red Macroalga, Sarcodia suiae. Biol Trace Elem Res 195, 291–300 (2020). https://doi.org/10.1007/s12011-019-01833-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01833-0