Abstract
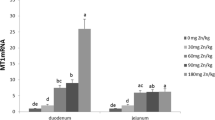
From the time of dietary intake to their utilization, the number of important interactions occurs among mineral elements, which can affect their bioavailability because of similarity in physicochemical properties and common absorptive pathways. However, the studies that have analyzed the interactions among copper, iron, and zinc have conflicting results and need further exploration. HT-29 cells grown to confluence in 6-well plates were incubated with increasing concentrations (0 to 200 μM) of Cu, Fe, and Zn for 3 and 6 h for uptake studies. Interaction studies involved measuring the uptake of metal in the presence of 0:1–4:1 ratio of the other metal for 3 h using atomic absorption spectrophotometer. The concentration of metal biomarkers and cytokines was also measured in the cell lysate following extracellular supplementation. The presence of 50 μM Zn significantly decreased (P < 0.05) cellular Cu uptake in HT-29 cells at 0.5:1 Cu:Zn ratio and also the cellular Fe uptake at the ratios 0.5:1, 2:1, and 4:1 Fe:Zn. The presence of 50 μM Fe significantly (P < 0.05) decreased cellular Cu uptake at the ratios 1:1, 2:1, and 4:1 Cu:Fe. The concentration of metallothionein responded significantly (P < 0.05) to changes in extracellular Zn concentration (supplementation and depletion). There was a decrease in concentration of IL-1β and TNF-α (P < 0.05) with an increasing extracellular concentration of Cu and Fe. The results of the study indicated that the presence of one mineral in the diet and multi mineral supplement may influence the bioavailability of the other mineral. Copper and iron may find application in promoting gut health.





Similar content being viewed by others
References
Windisch W (2002) Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal Bioanal Chem 372:421–425
Sandstorm B (2001) Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr 85(2):S181–S185
Troost FJ, Brummer RJM, Dainty JR, Hoogewerff JA, Bull VJ, Saris WHM (2003) Iron supplements inhibit zinc but not copper absorption in vivo in ileostomy subjects. Am J Clin Nutr 78:1018–1023
Fogh J, Trempe G (1975) New human tumor cell lines. In: Fogh J (ed) Human tumor cell in vitro, 1st edn. Springer, New York, pp 115–159
Lenaerts K, Bouwman F, Lamers W, Renes J, Mariman E (2007) Comparative proteomic analysis of cell lines and scrapings of the human intestinal epithelium. BMC Genomics 8:91
Glahn RP, Lee OA, Yeung A, Goldman MI, Miller DD (1998) Caco-2 cell ferritin formation predicts nonradiolabelled food iron availability in an in vitro digestion/ Caco-2 cell culture model. J Nutr 128:1555–1561
Davis SR, Cousins RJ (2000) Metallothionein expression in animals: a physiological perspective on function. J Nutr 130:1085–1088
Maret W, Krezel A (2007) Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med 13(7 –8):371–375
Michalczyk AA, Ackland ML (2013) hZip 1 (HSLC39A1) regulates zinc homeostasis in gut epithelial cells. Genes Nutr 8(5):475–486
Heyman M (2001) Symposium on dietary influences on mucosal immunity. How dietary antigens access the mucosal immune system. Proc Nutr Soc 60(4):419–426
McGee DW (1999) Inflammation and mucosal cytokine production. In: Ogra PL, Mestecky J, Lamm M, Strober W, Bienenstock J (eds) . Academic Press, Mucosal Immunity, pp 559–573
Sun M, He C, Cong Y, Liu Z (2015) Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol 8(5):969–978
Shanahan F (2001) Inflammatory bowel disease: immunodiagnostics, immunotherapeutics and ecotherapeutics. Gastroenterol 20(3):622–635
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347:417–429
Foster SL, Richardson SH, Failla ML (2001) Elevated iron status increases bacterial invasion and survival and alters cytokine/chemokine mRNA expression in Caco-2 human intestinal cells. J Nutr 131:1452–1458
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Kambe T, Weaver B, Andrews G (2008) The genetics of essential metal homeostasis during development. Genesis 46:214–228
Nishito Y, Kambe T (2018) Absorption mechanisms of Iron, copper, and zinc: an overview. J Nutr Sci Vitaminol 64:1–7
King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ (2016) Biomarkers of nutrition for development (BOND)-zinc review. J Nutr 146:858S–885S
Ozden TA, Gokçay G, Cantez MS, Durmaz O, Işsever H, Omer B, Saner G (2015) Copper, zinc and iron levels in infants and their mothers during the first year of life: a prospective study. BMC Pediatr 15:157
Reeves PG, Briske-Anderson M, Johnson L (1998) Physiological concentrations of zinc affect the kinetics of copper uptake and transport in the human intestinal cell model, Caco-2. J Nutr 128:1794–1801
Arredondo M, Martinez R, Nunez MT, Ruz M, Olivares M (2006) Inhibition of iron and copper uptake by iron, copper and zinc. Biol Res 39(1):95–102
Ha JH, Doguer C, Wang X, Flores SR, Collins JF (2016) High iron consumption impairs growth and causes copper deficiency anaemia in weanling Sprague Dawley rats. PLoS One 11(8):1–19
Fox PL (2003) The copper-iron chronicles: the story of an intimate relationship. Biometals 16(1):9–40
Ravia JJ, Stephen RM, Ghishan FK, Collins JF (2005) Menkes copper ATPase (Atp7a) is a novel metalresponsive gene in rat duodenum, and immunoreactive protein is present on brush border and basolateral membrane domains. J Biol Chem 280(43):36221–36227
Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF (2013) Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr 143(12):1927–1933
Tennant JP, Jai T, Sharp PA (2004) Mechanisms involved in copper uptake by human intestinal epithelial cells. Proc Nutr Soc 63:35A
Donangelo CM, Woodhouse LR, King SM, Viteri FE, King JC (2002) Supplemental zinc lowers measures of iron status in young women with low iron reserves. J Nutr 32(7):1860–1864
Rossander-Hultén L, Brune M, Sandström B, Lonnerdal B, Hallberg L (1991) Competitive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr 54:152–156
Iyengar V, Pullakhandam R, Nair KM (2009) Iron-zinc interaction during uptake in human intestinal Caco-2 cell line: kinetic analysis and possible mechanism. Indian J Biochem Biophys 46:299–306
Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR (2017) Zinc and oxidative stress: current mechanisms. Antioxidants (Basel) 6(2):24
Grungreiff K, Reinhold D, Wedemeyer H (2016) The role of zinc in liver cirrhosis. Ann Hepatol 15(1):7–16
Moltedo O, Verde C, Capasso A, Parisi E, Remondelli P, Bonatti S, Alvarez-Hernandez X, Glass J, Alvino CG, Leone A (2000) Zinc transport and metallothionein secretion in the intestinal human cell line Caco-2. J Biol Chem 275(41):31819–31825
Fosset C, Danzeisen R, Gambling L, McGaw BA, McArdle HJ (2009) Cu loading alters expression of non-IRE regulated, but not IRE regulated Fe dependent proteins in HepG2 cells. J Inorg Biochem 103:709–716
Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF (2011) Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 118:3146–3153
Hwang C, Ross V, Mahadevan U (2012) Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm Bowel Dis 18:1961–1981
Filippi J, Al-Jaouni R, Wiroth JB, Hebuterne X, Schneider SM (2006) Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis 12:185–191
Bao B, Prasad AS, Beck FW, Godmere M (2003) Zinc modulates mRNA levels of cytokines. Am J Phys 285:E1095–E1102
Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ (2004) Abrogation of nuclear factor-ƙB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-α. Am J Pathol 164:1547–1556
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:1–10
Acknowledgments
The authors are highly grateful to Indian Council of Medical Research, Department of Science & Technology and Defence Research and Developmental Organisation (DRDO), for award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakhra, G., Masih, D., Vats, A. et al. Study of Metal-Metal Interactions and Their Biomarkers Using an Intestinal Human Cell Line. Biol Trace Elem Res 195, 95–104 (2020). https://doi.org/10.1007/s12011-019-01831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01831-2