Abstract
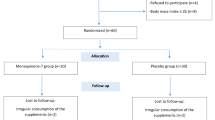
Metabolic failure is associated with dyslipidemia and coagulation which can result in a higher risk of cardiovascular disease (CVD) in type 2 diabetes mellitus (T2DM). The aim of this study was to assess the effects of choline and magnesium co-supplementation on lipid profile and coagulation parameters in patients with T2DM. In a randomized, double-blind, placebo-controlled trial, supplements of choline bitartrate (1000 mg), magnesium oxide (500 mg), choline plus magnesium, or placebo were administered for 2 months to 96 diabetic participants of both sexes aged 30–60 years. Anthropometric characteristics, dietary intake, physical activity, serum lipids, and coagulation markers were measured in all subjects. Significant differences were observed in plasminogen activator inhibitor-1 (PAI-1) levels in the magnesium and choline-magnesium groups (p < 0.05). Moreover, tissue plasminogen activator (tPA) levels increased in choline-magnesium groups (p < 0.001). When adjusted for potential confounders, a significant decrease in PAI-1 (p = 0.03) and a marginally significant increase in tPA (p = 0.054) were found in the choline-magnesium group compared with the other groups. Compared with baseline values, there were significant differences in serum magnesium, HDL, and triglycerides (TG) following choline-magnesium co-supplementation (p < 0.05); however, there were no significant differences in serum magnesium, HDL, and TG among the groups (p > 0.05). Overall, concurrent supplementation of magnesium and choline is more effective than either magnesium or choline alone to improve coagulation in subjects with T2DM.

Similar content being viewed by others
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87(1):4–14
Nia BH, Khorram S, Rezazadeh H, Safaiyan A, Tarighat-Esfanjani A (2018) The effects of natural clinoptilolite and nano-sized clinoptilolite supplementation on glucose levels and oxidative stress in rats with type 1 diabetes. Can J Diabetes 42(1):31–35
Shi Y, Hu FB (2014) The global implications of diabetes and cancer. Lancet 383(9933):1947–1948
Namazi N, Tarighat A, Bahrami A (2012) The effect of hydro alcoholic nettle (Urtica dioica) extract on oxidative stress in patients with type 2 diabetes: a randomized double-blind clinical trial. Pak J Biol Sci 15(2):98–102
Watson RR, Preedy VR (2012) Bioactive food as dietary interventions for diabetes, First edn, Elsevier, United Kingdom and United States of America.
Esser N, Paquot N, Scheen AJ (2015) Inflammatory markers and cardiometabolic diseases. Acta Clin Belg 70(3):193–199
Kulwas A, Lisewska B, Jundziłł W, Ruszkowska B, Drewniak W, Ruprecht Z et al (2017) Tissue plasminogen activator (t-PA) and plasminogen activator inhibitor type 1 (PAI-1) in diabetic foot syndrome. Adv Med Sci 62(1):87–91
Mortensen LS, Thomsen C, Hermansen K (2010) Effects of different protein sources on plasminogen inhibitor-1 and factor VII coagulant activity added to a fat-rich meal in type 2 diabetes. Rev Diabet Stud 7(3):233
Yarmolinsky J, Barbieri NB, Weinmann T, Ziegelmann PK, Duncan BB, Schmidt MI (2016) Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Sci Rep 6:17714
Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C (2008) Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr 87(2):424–430
EFSA Panel on Dietetic Products N, Allergies (2016) Dietary reference values for choline. EFSA J 14(8):e04484
Wiedeman A, Barr S, Green T, Xu Z, Innis S, Kitts D (2018) Dietary choline intake: current state of knowledge across the life cycle. Nutrients 10(10):1513
Wallace JM, McCormack JM, McNulty H, Walsh PM, Robson PJ, Bonham MP et al (2012) Choline supplementation and measures of choline and betaine status: a randomised, controlled trial in postmenopausal women. Br J Nutr 108(7):1264–1271
Mehta AK, Singh BP, Arora N, Gaur SN (2010) Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 215(7):527–534
Zeisel SH, Da Costa K-A (2009) Choline: an essential nutrient for public health. Nutr Rev 67(11):615–623
Yilmaz Z, Ozarda Y, Cansev M, Eralp O, Kocaturk M, Ulus IH (2010) Choline or CDP-choline attenuates coagulation abnormalities and prevents the development of acute disseminated intravascular coagulation in dogs during endotoxemia. Blood Coagul Fibrinolysis 21(4):339–348
Heidarianpour A, Sadeghian E, Gorzi A, Nazem F (2011) The influence of oral magnesium sulfate on skin microvasculature blood flow in diabetic rats. Biol Trace Elem Res 143(1):344–350
Bae Y-J, Choi M-K (2011) Magnesium intake and its relevance with antioxidant capacity in Korean adults. Biol Trace Elem Res 143(1):213–225
Wu J, Xun P, Tang Q, Cai W, He K (2017) Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Nutr J 16(1):60
Ozcaliskan Ilkay H, Sahin H, Tanriverdi F, Samur G (2018) Association between magnesium status, dietary magnesium intake, and metabolic control in patients with type 2 diabetes mellitus. J Am Coll Nutr:1–8
Barbagallo M, Dominguez LJ (2015) Magnesium and type 2 diabetes. World J Diabetes 6(10):1152
Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z (2018) Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 181(2):207–215
Verma H, Garg R (2017) Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: a systematic review and meta-analysis. J Hum Nutr Diet 30(5):621–633
Bo S, Pisu E (2008) Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol 19(1):50–56
Harnett MJ, Datta S, Bhavani-Shankar K (2001) The effect of magnesium on coagulation in parturients with preeclampsia. Anesth Analg 92(5):1257–1260
Jaffe R (2013) Phytonutrients in diabetes management, In: Bioactive food as dietary interventions for diabetes. First edn, Elsevier, United Kingdom and United States of America, pp 339–353
Medina D, David K, Lin Y, Schaar D, Patel V, Gharibo M et al (2017) Choline-magnesium trisalicylate modulates acute myelogenous leukemia gene expression during induction chemotherapy. Leuk Lymphoma 58(5):1227–1230
Moffett BS, Arora G, Bricker JT (2003) Treatment of acute rheumatic carditis with choline magnesium trisalicylate in a patient needing surgery. J Pediatr Pharmacol Ther 8(4):284–286
Strair RK, Gharibo M, Schaar D, Rubin A, Harrison J, Aisner J et al (2008) Nuclear factor-κB modulation in patients undergoing induction chemotherapy for acute myelogenous leukemia. Clin Cancer Res 14(22):7564–7568
Rashvand S, Mobasseri M, Tarighat-Esfanjani A (2019) The effects of choline and magnesium co-supplementation on metabolic parameters, inflammation, and endothelial dysfunction in patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. J Am Coll Nutr:1–8
Belalcazar LM, Anderson AM, Lang W, Schwenke DC, Haffner SM, Yatsuya H et al (2014) Fiber intake and plasminogen activator inhibitor-1 in type 2 diabetes: look AHEAD (Action for Health in Diabetes) trial findings at baseline and year 1. J Acad Nutr Diet 114(11):1800–1810 e2
Committee IR (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms. https://www.IPAQ.ki.se. Accessed 17 Sept 2005:2008
Hongu N, Sachan DS (2003) Carnitine and choline supplementation with exercise alter carnitine profiles, biochemical markers of fat metabolism and serum leptin concentration in healthy women. J Nutr 133(1):84–89
Effects of oral magnesium for migraine prophylaxis, Pharmaceutical Sciences, 15(1): 103- 108
SCF, Scientific Committee of Food (2002) Opinion of the scientific committee of food on the tolerable upper intake levels of magnesium. European Commission, https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out122_en.pdf. Accessed 28 Feb 2002
McBane RD II, Hardison RM, Sobel BE, Group BDS (2010) Comparison of plasminogen activator inhibitor-1, tissue type plasminogen activator antigen, fibrinogen, and D-dimer levels in various age decades in patients with type 2 diabetes mellitus and stable coronary artery disease (from the BARI 2D trial). Am J Cardiol 105(1):17–24
Belalcazar LM, Ballantyne CM, Lang W, Haffner SM, Rushing J, Schwenke DC et al (2011) Metabolic factors, adipose tissue, and plasminogen activator inhibitor-1 levels in type 2 diabetes: findings from the look AHEAD study. Arterioscler Thromb Vasc Biol 31(7):1689–1695
Borisevich N, Loznikova S, Sukhodola A, Halets I, Bryszewska M, Shcharbin D (2013) Acidosis, magnesium and acetylsalicylic acid: effects on thrombin. Spectrochim Acta A Mol Biomol Spectrosc 104:158–164
Nadler JL, Malayan S, Luong H, Shaw S, Natarajan RD, Rude RK (1992) Intracellular free magnesium deficiency plays a key role in increased platelet reactivity in type II diabetes mellitus. Diabetes Care 15(7):835–841
Na H, Shin H, Kang S, Hwang J, Do S (2014) Effects of magnesium sulphate on coagulation after laparoscopic colorectal cancer surgery, measured by rotational thromboelastometry (ROTEM®). Anaesthesia 69(12):1314–1321
Stewart D, Marder V, Starkman S, Saver J (2006) Magnesium sulfate neither potentiates nor inhibits tissue plasminogen activator-induced thrombolysis. J Thromb Haemost 4(7):1575–1579
Shahbah D, Hassan T, Morsy S, El Saadany H, Fathy M, Al-Ghobashy A et al (2017) Oral magnesium supplementation improves glycemic control and lipid profile in children with type 1diabetes and hypomagnesaemia. Medicine 96(11):1-6
Hadjistavri LS, Sarafidis PA, Georgianos PI, Tziolas IM, Aroditis CP, Hitoglou-Makedou A et al (2010) Beneficial effects of oral magnesium supplementation on insulin sensitivity and serum lipid profile. Med Sci Monit 16(6):CR0–CCR
Sivanesan S, Taylor A, Zhang J, Bakovic M (2018) Betaine and Choline Improve Lipid Homeostasis in Obesity by Participation in Mitochondrial Oxidative Demethylation, Frontiers in Nutrition, 5:1-16. https://doi.org/10.3389/fnut.2018.00061
Jack-Roberts C, Joselit Y, Nanobashvili K, Bretter R, Malysheva O, Caudill M et al (2017) Choline supplementation normalizes fetal adiposity and reduces lipogenic gene expression in a mouse model of maternal obesity. Nutrients 9(8):899
Ilcol YO, Yilmaz Z, Cansev M, Ulus IH (2009) Choline or CDP-choline alters serum lipid responses to endotoxin in dogs and rats: involvement of the peripheral nicotinic acetylcholine receptors. Shock 32(3):286–294
Ueland PM (2011) Choline and betaine in health and disease. J Inherit Metab Dis 34(1):3–15
Simental-Mendía LE, Simental-Mendía M, Sahebkar A, Rodríguez-Morán M, Guerrero-Romero F (2017) Effect of magnesium supplementation on lipid profile: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 73(5):525–536
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashvand, S., Mobasseri, M. & Tarighat-Esfanjani, A. Effects of Choline and Magnesium Concurrent Supplementation on Coagulation and Lipid Profile in Patients with Type 2 Diabetes Mellitus: a Pilot Clinical Trial. Biol Trace Elem Res 194, 328–335 (2020). https://doi.org/10.1007/s12011-019-01802-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01802-7