Abstract
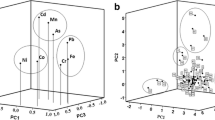
This study reports the simultaneous determination of the total concentrations of Al, Ca, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P, and Zn in 17 samples of commercial energy drinks through inductively coupled plasma optical emission spectrometry and multivariate methods, such as Pearson correlation and principal component analysis (PCA), in order to conduct a more thorough evaluation of the original data. The samples studied were stored in two types of containers (polyethylene terephthalate bottles and aluminum cans) and purchased in the city of Belém (State of Pará, Brazil). The results showed high Na content in energy drinks, followed by K, Ca, and Mg. The accuracy of the optimized method was evaluated with the certified reference materials to assess trace elements in water (NIST 1643e); the resultant recoveries varied from 83 to 105%. Energy drinks stored in cans presented higher levels of aluminum and magnesium, while those bottled in polyethylene terephthalate bottles had a higher K content. There were significant differences between the observed Na concentrations and the values dictated on the drink package. Furthermore, PCA explained 70.38% of the total variance, allowing for an evaluation of the degree of similarity between the energy drinks studied and showing that the main contributions to the formation of groups are related to Fe, Na, Mg, and Zn contents. These results will be used to better understand the distribution of inorganic elements contained in energy drinks.

Similar content being viewed by others
References
Bulut B, Beyhun NE, Topbaş M, Çan G (2014) Energy drink use in university students and associated factors. J Community Health 39:1004–1011. https://doi.org/10.1007/s10900-014-9849-3
Arria AM, Bugbee BA, Caldeira KM, Vincent KB (2014) Evidence and knowledge gaps for the association between energy drink use and high-risk behaviors among adolescents and young adults. Nutr Rev 72:87–97. https://doi.org/10.1111/nure.12129
Emond JA, Gilbert-Diamond D, Tanski SE, Sargent JD (2014) Energy drink consumption and the risk of alcohol use disorder among a national sample of adolescents and young adults. J Pediatr 165:1194–1200. https://doi.org/10.1016/j.jpeds.2014.08.050
Heckman MA, Sherry K, Gonzalez De Mejia E (2010) Energy drinks: an assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf 9:303–317. https://doi.org/10.1111/j.1541-4337.2010.00111.x
Szymczycha-Madeja A, Welna M, Pohl P (2013) Determination of elements in energy drinks by ICP OES with minimal sample preparation. J Braz Chem Soc 24(10):1606–1612. https://doi.org/10.5935/0103-5053.20130202
Rath M, BSN, FNP-s (2012) Energy drinks: what is all the hype? The dangers of energy drink consumption. J Am Acad Nurse Pract 24:70–76. https://doi.org/10.1111/j.1745-7599.2011.00689.x
Wiklund U, Karlsson M, Öström M, Messner T (2009) Influence of energy drinks and alcohol on post-exercise heart rate recovery and heart rate variability. Clin Physiol Funct Imaging 29:74–80. https://doi.org/10.1111/j.1475-097X.2008.00837.x
Reissig CJ, Strain EC, Griffiths RR (2009) Caffeinated energy drinks—a growing problem. Drug Alcohol Depend 99:1–10. https://doi.org/10.1016/j.drugalcdep.2008.08.001
Franco F (1998) Tabela de composição de alimentos, 9th edn. Atheneu, São Paulo
Harper HA, Mayes RA (1982) Manual de Química fisiologica, 5th edn. Atheneu, São Paulo
Massey R, Taylor S (1991) Aluminium in food and the environment. Royal Society of Chemistry, London
Tomljenovic L (2011) Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis 23:567–598. https://doi.org/10.3233/JAD-2010-101494
Uauy R, Olivares M, González M (1998) Essentiality of copper in humans. Am J Clin Nutr 67:952–959. https://doi.org/10.1093/ajcn/67.5.952S
Bothwell T, H; Charlton RW, Cook JD, Finch CA (1979) Iron metabolism in man. Blackwell Scientific, Oxford
Siegmund B, Derler K, Pfannhauser W (2004) Chemical and sensory effects of glass and laminated carton packages on fruit juice products – still a controversial topic. Food Sci Technol 37:481–488. https://doi.org/10.1016/j.lwt.2003.11.005
Jellesen MS, Rasmussen AA, Hilbert LR (2006) A review of metal release in the food industry. Mater Corros 57(5):387–393. https://doi.org/10.1002/maco.200503953
Castro MTPO, Baccan N (2005) Application of factorial design in optimization of preconcentration procedure for copper determination in soft drink by flame atomic absorption spectrometry. Talanta 65:1264–1269. https://doi.org/10.1016/j.talanta.2004.09.002
Nascentes CC, Kamogawa MY, Fernandes KG, Arruda MAZ, Nogueira ARA, Nóbrega JA (2005) Direct determination of Cu, Mn, Pb and Zn in beer by thermospray flame furnace atomic absorption spectrometry. Spectrochim Acta B 60:749–753. https://doi.org/10.1016/j.sab.2005.02.012
Schiavo D, Neira JY, Nóbrega JA (2008) Direct determination of Cd, Cu and Pb in wines and grape juices by thermospray flame furnace atomic absorption spectromety. Talanta 76:1113–11118. https://doi.org/10.1016/j.talanta.2008.05.010
Sweileh JA, Misef KY, El-Sheikh AH, Sunjuk MS (2014) Development of a new method for determination of aluminum (Al) in Jordanian foods and drinks: solid phase extraction and adsorption of Al3+-D-mannitol on carbon nanotubes. J Food Compos Anal 33:6–13. https://doi.org/10.1016/j.jfca.2013.10.002
de Amorim FR, Bof C, Franco MB, da Silva JBB, Nascentes CC (2006) Comparative study of conventional and multivariate methods for aluminum determination in soft drinks by graphite furnace atomic absorption spectrometry. Microchem J 82:168–173. https://doi.org/10.1016/j.microc.2006.01.011
Alkıs IM, Öz S, Atakol A, Yılmaz N, Anlı RE, Atakol O (2014) Investigation of heavy metal concentrations in some Turkish wines. J Food Compos Anal 33:105–110. https://doi.org/10.1016/j.jfca.2013.11.006
Francisco BBA, Brum DM, Cassella RJ (2015) Determination of metais in soft drinks packed in different materials by ETAAS. Food Chem 185:488–494. https://doi.org/10.1016/j.foodchem.2015.04.020
Ozbek N, Akman S (2015) Determination of boron in Turkish wines by microwave plasma atomic emission spectrometry. LWT Food Sci Technol 61:532–535. https://doi.org/10.1016/j.lwt.2014.11.047
Froes RES, Borges Neto W, Naveira RLP, Silva NC, Nascentes CC, Silva JBB (2009) Exploratory analysis and inductively coupled plasma optical emission spectrometry (ICP OES) applied in the determination of metals in soft drinks. Microchem J 92:68–72. https://doi.org/10.1016/j.microc.2008.12.008
Bingol M, Yentur G, Er B, Oktem AB (2010) Determination of some heavy metal levels in soft drinks from Turkey using ICP-OES method. Czech J Food Sci 28(3):213–216. https://doi.org/10.17221/158/2008-CJFS
Leśniewicz A, Grzesiak M, Żyrnicki W, Borkowska-Burnecka J (2016) Mineral composition and nutritive value of isotonic and energy drinks. Biol Trace Elem Res 170:485–495. https://doi.org/10.1007/s12011-015-0471-8
Nkono NA, Asubioj OI (1997) Trace elements in bottled and soft drinks in Nigeria – a preliminary study. Sci Total Environ 208:161–163. https://doi.org/10.1016/S0048-9697(97)00289-1
Kılıç S, Yenisoy-Karakaş S, Kılıç M (2015) Metal contamination in fruit juices in Turkey: method validation and uncertainty budget. Food Anal Methods 8:2487–2495. https://doi.org/10.1007/s12161-015-0136-4
Zucchi OLAD, Moreira S, Salvador MJ, Santos LL (2005) Multielement analysis of soft drinks by X-ray fluorescence spectrometry. J Agric Food Chem 53:7863–7869. https://doi.org/10.1021/jf0510945
Mingoti SA (2005) Análise de Dados através de Métodos de Estatística Multivariada. Editora UFMG, Belo Horizonte
Beebe KR, Pell RJ, Seasholtz MB (1998) Chemometrics: a practical guide. Wiley, New York
de Barros Neto B, Scarminio IS, Bruns RE (2006) 25 anos de quimiometria no Brasil. Quim Nova 29:1401–1406. https://doi.org/10.1590/S0100-40422006000600042
Ferreira MMC, Antunes AM, Melgo MS, Volpe PLO (1999) Quimiometria I: calibração multivariada, um tutorial. Quim Nova 22:724–731. https://doi.org/10.1590/S0100-40421999000500016
Hair JF, Tathan RL, Anderson RE (2005) Análise multivariada de dados, 5a edn. Bookman, Porto Alegre
Thomsen V, Roberts G, Burguess K (2000) The concepts of background equivalent concentration in spectroscopy. Spectroscopy 15:33
Rexan Beverage Can Americas SA (2004) Descritivo de processo. São Paulo, Brazil
ANVISA (2003) Technical regulation on food labeling. Resolution - RDC n° 360, December 23, 2003. Retrieved june 12, 2017 from: http://www.abic.com.br/publique/media/CONS_leg_resolucao360-03.pdf
Shun-Xing L, Lu-Xiu L, Jing L, Feng-Ying Z, Qing-Xiang W, Wen W (2010) Speciation analysis, bioavailability and risk assessment of trace metals in herbal decoctions using a combined technique of in vitro digestion and biomembrane filtration as sample pretreatment method. Phytochem Anal 21:590–596. https://doi.org/10.1002/pca.1239
Otten JJ, Hellwig JP, Meyers LD (2006) Dietary reference intakes. The essential guide to nutrient requirements. The National Academies Press: Washington, USA. Retrieved June 12, 2017 from: https://www.nal.usda.gov/sites/default/files/fnic_uploads/DRIEssentialGuideNutReq.pdf
WHO (1996) Trace elements in human nutrition and health, Geneva. Retrieved June 30, 2017 from: www.who.int
WHO (2011). Guidelines for drinking water quality. 4th edition, Geneva, Switzerland, 307–442. Retrieved June 30, 2017 from: www.who.int
Carvalho FIM, Dantas Filho HA (2014) Estudo da qualidade da gasolina tipo A e sua composição química empregando análise de componentes principais. Quim Nova 37:33–38. https://doi.org/10.1590/S0100-40422014000100007
Vandeginste BGM, Massart DL, Buydens LMC, De Jong S, Lewi PJ, Smeyers-Verbeke J (1998) Handbook of chemometrics and qualimetrics: Part b. Elsevier, Amsterdam
Funding
This research was funded by Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA). J.B.P.J was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, A.S., Junior, J.B.P., de Araújo Gomes, A. et al. Mineral Composition Evaluation in Energy Drinks Using ICP OES and Chemometric Tools. Biol Trace Elem Res 194, 284–294 (2020). https://doi.org/10.1007/s12011-019-01770-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01770-y