Abstract
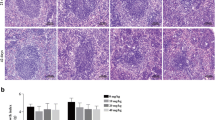
Zinc (Zn) is an essential trace element for animals. Zn controls the action of more than 300 enzymes and plays an important role in the regulation of gene expression. Evidence has shown that Zn has an antioxidant function, and oxidative damage can occur with Zn deficiency. To assess the effect of Zn deficiency–induced spleen fibrosis, Zn-deficient mice, normal mice, and high-Zn mice were generated and assessed. The Zn content of the spleen in each group was determined, and histopathological examination of the spleens of each group was performed. In the film, we found that the spleens of the Zn-deficient group had high levels of proteinaceous material exudation, interstitial broadening, and lymphocyte reduction, with increased collagen, α-SMA expression, antioxidants, and oxygen free radicals. Zn deficiency inhibited the expression of antioxidants in mice, and the activity of oxygen free radicals in Zn-deficient mice was increased. The detection of α-SMA, collagen 1, and TGF-β by fluorescence quantitative PCR revealed that the expression index increased in Zn-deficient mice. In addition, to verify the effect of Zn deficiency on the extracellular matrix (ECM) regulatory system, MMPs were determined by real-time PCR, and the expression in the Zn deficiency group was lower than that in the normal group and high-Zn group. The MMP-2 and MMP-13 analyses showed that the expression of the high-Zn group was significantly higher than that of the normal group, indicating that Zn plays an important role in its expression. The above experimental analysis showed that Zn deficiency induces oxygen free radical damage, which further leads to spleen fibrosis.





Similar content being viewed by others
References
Ohashi W, Fukada T (2019) Contribution of zinc and zinc transporter in the pathogenesis of inflammatory bowel diseases. J Immunol Res 2019:8396878
Vallee BL, Falchuk KH (1993) The biochemical basis of Zn physiology. Physiol Rev 73(1):79–118
Choi S-H, Lee K-L, Shin J-H, Cho Y-B, Cha S-S, Roe J-H (2017) Zn-dependent regulation of Zn import and export genes by Zur. Nat Commun 8:15812
Sun Q, Zhong W, Zhang W, Zhou Z (2016) Defect of mitochondrial respiratory chain is a mechanism of ROS overproduction in a rat model of alcoholic liver disease: role of Zn deficiency. Am J Physiol Gastrointest Liver Physiol 310(3):G205–G214
Prasad AS (2012) Discovery of human Zn deficiency: 50 years later. J Trace Elem Med Biol 26(2–3):66–69
Sakiyama H, Fujiwara N, & Yoneoka Y, et al. (2016) Cu,Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radical Res 50:666-77
Xu JM, Xi JK, Xu ZL (2016) Injurious effect of Zn deficiency on cardiomyocytes. Sheng Li Xue Bao 68(5):677–683
Bin B-H, Seo J, Kim ST (2018) Function, structure, and transport aspects of ZIP and ZnT Zn transporters in immune cells. J Immunol Res 2018:9365747
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: Zn and metallothioneins. Biomed Pharmacother 57(9):399–411
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zn and human health: an update. Arch Toxicol 86(4):521–534
Kerr ME, Bender CM, Monti EJ (1996) An introduction to oxygen free radicals. Heart Lung 25(3):200–209 quiz 210-1.
Purohit V, Brenner DA (2006) Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology 43(4):872–878
Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM (2008) Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44(7):1259–1272
Leonarduzzi G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, Dargel R, Poli G (1997) The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta1 expression in the macrophage lineage: a link between oxidative injury and fibrosclerosis. FASEB J 11(11):851–857
Richeldi L, Collard HR, Jones MG (2017) Idiopathic pulmonary fibrosis. Lancet 389(10082):1941–1952
Misharin AV, Morales-Nebreda RPA, Cuda CM et al (2017) Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 214(8):2387–2404
Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N (2017) Management strategies for liver fibrosis. Ann Hepatol 16(1):48–56
Guo H, Bi X, Zhou P, Zhu S, Ding W (2017) NLRP3 deficiency attenuates renal fibrosis and ameliorates mitochondrial dysfunction in a mouse unilateral ureteral obstruction model of chronic kidney disease. Mediat Inflamm 2017:8316560
Prakash J, Pinzani M (2017) Fibroblasts and extracellular matrix: targeting and therapeutic tools in fibrosis and cancer. Adv Drug Deliv Rev 121:1–2
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27
Ding Y, Choi ME (2014) Regulation of autophagy by TGF-β: emerging role in kidney fibrosis. Semin Nephrol 34(1):62–71
Ignotz RA, Massague J (1986) Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261(9):4337–4345
Edwards DR, Murphy G, Reynolds J, Whitham S, Docherty A et al (1987) Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J 6(7):1899–1904
Arpino V, Brock M, Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44–46:247–254
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzym Inhib Med Ch 31(1):177–183
Gotardo ÉMF, Santos PS, Acedo SC, de Morais TR, Ribeiro ML, Gambero A (2017) Extracellular matrix remodeling and matrix metalloproteinase inhibition in visceral adipose during weight cycling in mice. Exp Cell Res 359(2):431–440
Ohbayashi H (2002) Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci 3(4):409–421
Mebius RE, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5(8):606–616
Tarantino G, Savastano S, Capone D, Colao A (2011) Spleen: a new role for an old player? World J Gastroenterol 17(33):3776–3784
Cesta MF (2006) Normal structure, function, and histology of the spleen. Toxicol Pathol 34(5):455–465
Lori A, Perrotta M, Lembo G, Carnevale D (2017) The spleen: a hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int J Mol Sci 18 (6).
Border WA, Noble NA (1998) Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int 54(4):1390–1391
Liu R-M, Desai LP (2015) Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol 6:565–577
Liu R-M, Pravia KG (2010) Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med 48(1):1–15
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 31502130) and the National Students innovation and entrepreneurship training program for college students of Huazhong Agricultural University (No. 201710504008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, Hx., Chen, Y. et al. Zinc Deficiency Induces Oxidative Damage and Causes Spleen Fibrosis. Biol Trace Elem Res 194, 203–209 (2020). https://doi.org/10.1007/s12011-019-01762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01762-y