Abstract
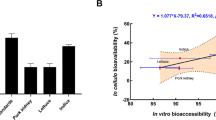
Investigating the bioaccessibility and bioavailability of Cd based on real contaminated cooked rice matrixes helps establish an accurate risk assessment method and effectively reduce the digestion and absorption of Cd. An 11-day in vitro rapid Caco-2/HT-29 co-culture cell model was used to establish and evaluate the simulation of the absorption and transport of Cd in the small intestine with a 70:30 Caco-2/HT-29 co-culture ratio and 1.0 mmol L−1 butyric acid as a differentiation inducer. The bioaccessibility and bioavailability of Cd in cooked rice were studied using the cell model combined with an in vitro digestion model. The bioaccessibility of Cd of each of the three cooked rice samples was significantly higher in the gastric phase (59.04–80.23%) than in the gastrointestinal phase (37.14–52.93%). Despite the extension of the digestion time of the gastrointestinal phase, no significant difference was found among the time points. Results demonstrated that the amount of undigested residue, not the level of Cd contamination, significantly contributed to the bioaccessibility of Cd, which was affected by pH or ion. The absorption rate of Cd (25.08% ± 3.05%) was greater than the values obtained using the pure Caco-2 cell models. The bioavailability of Cd (8.29% ± 1.95%) was almost similar to that of Zn2+ (6.66% ± 1.41%) in the cooked rice matrix, indicating that the intestinal epithelium expressed a strong absorptive capacity of Cd during the absorption of essential metallic elements. The 11-day rapid Caco-2/HT-29 co-culture cell model combined with the in vitro digestion model was an efficient tool for studying the bioaccessibility and bioavailability of Cd or other substances in a food matrix to further investigate mechanistic steps and screen a broad set of food matrix factors.










Similar content being viewed by others
References
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxid Med Cell Longevity 2013:898034. https://doi.org/10.1155/2013/898034
Bolam T, Bersuder P, Burden R, Shears G, Morris S, Warford L, Thomas B, Nelson P (2016) Cadmium levels in food containing crab brown meat: a brief survey from UK retailers. J Food Compos Anal 54:63–69. https://doi.org/10.1016/j.jfca.2016.10.005
Zhuang P, Zhang C, Li Y, Zou B, Mo H, Wu K, Wu J, Li Z (2016) Assessment of influences of cooking on cadmium and arsenic bioaccessibility in rice, using an in vitro physiologically-based extraction test. Food Chem 213:206–214. https://doi.org/10.1016/j.foodchem.2016.06.066
Nogawa K, Kobayashi E, Okubo Y, Suwazono Y (2004) Environmental cadmium exposure, adverse effects and preventive measures in Japan. BioMetals 17(5):581–587. https://doi.org/10.1023/B:BIOM.0000045742.81440.9c
Lei M, Tie BQ, Song ZG (2015) Heavy metal pollution and potential health risk assessment of white rice around mine areas in Hunan Province, China. Food Sec 7(1):45–54. https://doi.org/10.1007/s12571-014-0414-9
Nogawa K, Suwazono Y, Nishijo M, Sakurai M, Ishizaki M, Morikawa Y, Watanabe Y, Kido T, Nakagawa H (2018) Relationship between mortality and rice cadmium concentration in inhabitants of the polluted Jinzu River basin, Toyama, Japan: a 26 year follow-up. J Appl Toxicol 38:855–861. https://doi.org/10.1002/jat.3593
Kosolsaksakul P, Farmer JG, Oliver IW, Graham MC (2014) Geochemical associations and availability of cadmium (Cd) in a paddy field system, northwestern Thailand. Environ Pollut 187(8):153–161. https://doi.org/10.1016/j.envpol.2014.01.006
Ahn SC, Chang JY, Lee JS, Yu HY, Jung AR, Kim JY, Choi JW, Hong YS, Do Yu S, Choi K (2016) Exposure factors of cadmium for residents in an abandoned metal mine area in Korea. Environ Geochem Health 39(5):1059–1070. https://doi.org/10.1007/s10653-016-9872-7
Sharma S, Nagpal AK, Kaur I (2018) Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem 255:15–22. https://doi.org/10.1016/j.foodchem.2018.02.037
Chandler JD, Cherry W, Banton SA, Li S, Orr ML, Boyd BD et al (2016) Low-dose oral cadmium increases airway reactivity and lung neuronal gene expression in mice. Physiol Rep 4(13):e12821. https://doi.org/10.14814/phy2.12821
Nakamura Y, Ohba K, Suzuki K, Ohta H (2012) Health effects of low-level cadmium intake and the role of metallothionein on cadmium transport from mother rats to fetus. J Toxicol Sci 37(1):149–156. https://doi.org/10.2131/jts.37.149
Santana VP, Salles ÉS, Correa DE, Gonçalves BF, Campos SG, Justulin LA, Godinho AF, Scarano WR (2016) Long-term effects of perinatal exposure to low doses of cadmium on the prostate of adult male rats. Int J Exp Pathol 97(4):310–316. https://doi.org/10.1111/iep.12193
Wang W, Schaumberg DA, Park SK (2016) Cadmium and lead exposure and risk of cataract surgery in US adults. Int J Hyg Environ Health 219(8):850–856. https://doi.org/10.1016/j.ijheh.2016.07.012
Järup L, Akesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238(3):201–208. https://doi.org/10.1016/j.taap.2009.04.020
Sanders AP, Flood K, Shu C, Herring AH, Wolf L, Fry RC (2012) Towards prenatal biomonitoring in North Carolina: assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS One 7(3):e31354. https://doi.org/10.1371/journal.pone.0031354
Xie X, Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, Tian Y (2013) The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut 175(8):30–34. https://doi.org/10.1016/j.envpol.2012.12.013
Hinwood AL, Callan AC, Ramalingam M, Boyce M, Heyworth J, Mccafferty P et al (2013) Cadmium, lead and mercury exposure in non smoking pregnant women. Environ Res 126(4):118–124. https://doi.org/10.1016/j.envres.2013.07.005
Aziz R, Rafiq MT, Li T, Liu D, He Z, Stoffella PJ, Sun K, Xiaoe Y (2015) Uptake of cadmium by rice grown on contaminated soils and its bioavailability/toxicity in human cell lines (Caco-2/HL-7702). J Agric Food Chem 63(13):3599–3608. https://doi.org/10.1021/jf505557g
Lee SG, Kim J, Park H, Holzapfel W, Lee KW (2017) Assessment of the effect of cooking on speciation and bioaccessibility/cellular uptake of arsenic in rice, using in vitro digestion and Caco-2 and PSI cells as model. Food Chem Toxicol 111:597–604. https://doi.org/10.1016/j.fct.2017.11.052
Fu J, Cui Y (2013) In vitro digestion/Caco-2 cell model to estimate cadmium and lead bioaccessibility/bioavailability in two vegetables: the influence of cooking and additives. Food Chem Toxicol 59(9):215–221. https://doi.org/10.1016/j.fct.2013.06.014
Versantvoort CH, Oomen AG, Vand KE, Rompelberg CJ, Sips AJ (2005) Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem Toxicol 43(1):31–40. https://doi.org/10.1016/j.fct.2004.08.007
Omar NA, Praveena SM, Aris AZ, Hashim Z (2015) Health risk assessment using in vitro, digestion model in assessing bioavailability of heavy metal in rice: a preliminary study. Food Chem 188:46–50. https://doi.org/10.1016/j.foodchem.2015.04.087
Kabak B, Ozbey F (2012) Assessment of the bioaccessibility of aflatoxins from various food matrices using an in vitro, digestion model, and the efficacy of probiotic bacteria in reducing bioaccessibility. J Food Compos Anal 27(1):21–31. https://doi.org/10.1016/j.jfca.2012.04.006
Pan F, Han L, Zhang Y, Yu Y, Liu J (2015) Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int J Food Sci Nutr 66(6):680–685. https://doi.org/10.3109/09637486.2015.1077792
Mahler GJ, Shuler ML, Glahn RP (2009) Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J Nutr Biochem 20(7):494–502. https://doi.org/10.1016/j.jnutbio.2008.05.006
Perales S, Barberá R, Lagarda MJ, Farré R (2010) Bioavailability of zinc from infant foods by in vitro methods (solubility, dialyzability and uptake and transport by Caco-2 cells). J Sci Food Agric 86(6):971–978. https://doi.org/10.1002/jsfa.2445
Bodinier M, Legoux MA, Pineau F, Triballeau S, Segain JP, Brossard C, Denery-Papini S (2007) Intestinal translocation capabilities of wheat allergens using the Caco-2 cell line. J Agric Food Chem 55(11):4576–4583. https://doi.org/10.1021/jf070187e
Hilgers AR, Conradi RA, Burton PS (1990) Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm Res 7(9):902–910. https://doi.org/10.1023/A:1015937605100
Satake M, Enjoh M, Nakamura Y, Takano T, Kawamura Y, Arai S et al (2002) Transepithelial transport of the bioactive tripeptide, Val-Pro-Pro, in human intestinal Caco-2 cell monolayers. Biosci Biotechnol Biochem 66(2):378–384. https://doi.org/10.1271/bbb.66.378
Stuknytė M, Maggioni M, Cattaneo S, Luca PD, Fiorilli A, Ferraretto A et al (2015) Release of wheat gluten exorphins A5 and C5 during in vitro gastrointestinal digestion of bread and pasta and their absorption through an in vitro model of intestinal epithelium. Food Res Int 72:208–214. https://doi.org/10.1016/j.foodres.2015.04.002
Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B (2012) Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm 83(3):427–435. https://doi.org/10.1016/j.ejpb.2012.10.003
García-Rodríguez A, Vila L, Cortés C, Hernández A, Marcos R (2018) Exploring the usefulness of the complex in vitro intestinal epithelial model Caco-2/HT29/Raji-B in nanotoxicology. Food Chem Toxicol 113:162–170. https://doi.org/10.1016/j.fct.2018.01.042
Anouk K, André CM, Yves-Jacques S, Lucien H, Torsten B (2016) Carotenoid and polyphenol bioaccessibility and cellular uptake from plum and cabbage varieties. Food Chem 197(Pt A):325–332. https://doi.org/10.1016/j.foodchem.2015.10.049
Rocha RA, Vélez D, Devesa V (2012) In vitro evaluation of intestinal fluoride absorption using different cell models. Toxicol Lett 210(3):311–317. https://doi.org/10.1016/j.toxlet.2012.02.015
Volstatova T, Havlik J, Potuckova M, Geigerova M (2016) Milk digesta and milk protein fractions influence the adherence of lactobacillus gasseri R and Lactobacillus casei FMP to human cultured cells. Food Funct 7(8):3531–3538. https://doi.org/10.1039/C6FO00545D
Calatayud M, Vázquez M, Devesa V, Vélez D (2012) In vitro study of intestinal transport of inorganic and methylated arsenic species by Caco-2/HT29-MTX cocultures. Chem Res Toxicol 25(12):2654–2662. https://doi.org/10.1021/tx300295n
Schimpel C, Teubl B, Absenger M, Meindl C, Fröhlich E, Leitinger G, Zimmer A, Roblegg E (2014) Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol Pharm 11(3):808–818. https://doi.org/10.1021/mp400507g
Donetti E, Arnaboldi F, Cornaghi L, Luca PD, Maggioni M, Fiorilli A et al (2014) An innovative in vitro co-culture of Caco2 and HT-29 cells for mimicking human intestinal epithelium: a morphological analysis. Ital J Anat Embryol 119(1):75. https://doi.org/10.13128/IJAE-15912
Lentz KA, Hayashi J, Lucisano LJ, Polli JE (2000) Development of a more rapid, reduced serum culture system for Caco-2 monolayers and application to the biopharmaceutics classification system. Int J Pharm 200(1):41–51. https://doi.org/10.1016/S0378-5173(00)00334-3
Uchida M, Fukazawa T, Yamazaki Y, Hashimoto H, Miyamoto Y (2009) A modified fast (4 day) 96-well plate Caco-2 permeability assay. J Pharmacol Toxicol Methods 59(1):39–43. https://doi.org/10.1016/j.vascn.2008.10.006
Vivek G, Nishit D, Samir M (2013) Permeation of insulin, calcitonin and exenatide across Caco-2 monolayers: measurement using a rapid, 3-day system. PLoS One 8(2):e57136. https://doi.org/10.1371/journal.pone.0057136
Cai Y, Xu C, Chen P, Hu J, Hu R, Huang M, Bi H (2014) Development, validation, and application of a novel 7-day Caco-2 cell culture system. J Pharmacol Toxicol Methods 70(2):175–181. https://doi.org/10.1016/j.vascn.2014.07.001
Turco L, Catone T, Caloni F, Consiglio ED, Testai E (2011) C aco-2/TC7 cell line characterization for intestinal absorption: how reliable is this in vitro model for the prediction of the oral dose fraction absorbed in human? Toxicol. In Vitro 25:13–20. https://doi.org/10.1016/j.tiv.2010.08.009
Ferraretto A, Bottani M, Luca PD, Cornaghi L, Arnaboldi F, Maggioni M et al (2018) Morpho-functional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci Rep 38(2):BSR20171497. https://doi.org/10.13128/IJAE-15912
Sun S, Zhang H, Sun F, Zhao Y, Chai Y, Zhang G (2014) Intestinal transport of sophocarpine across the caco-2 cell monolayer model and quantification by LC/MS. Biomed Chromatogr 28:885–890. https://doi.org/10.1002/bmc.3195
Hilgendorf C, Spahn-Langguth H, Regårdh CG, Lipka E, Amidon GL, Langguth P (2000) Caco-2 versus caco-2/HT29-MTX co-cultured cell lines: permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J Pharm Sci 89(1):63–75. https://doi.org/10.1002/(SICI)1520-6017(200001)89:1%3C63::AID-JPS7%3E3.0.CO;2-6
Han L, Zhao Y, Yin L, Li R, Liang Y, Huang H, Pan S, Wu C, Feng M (2012) Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS PharmSciTech 13(3):836–845. https://doi.org/10.1208/s12249-012-9807-2
Béduneau A, Tempesta C, Fimbel S, Pellequer Y, Jannin V, Demarne F, Lamprecht A (2014) A tunable Caco-2/HT29-MTX co-culture model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur J Pharm Biopharm 87(2):290–298. https://doi.org/10.1016/j.ejpb.2014.03.017
Wood KM, Stone GM, Peppas NA (2010) The effect of complexation hydrogels on insulin transport in intestinal epithelial cell models. Acta Biomater 6(1):48–56. https://doi.org/10.1016/j.actbio.2009.05.032
Aziz R, Rafiq MT, Yang J, Liu D, Lu L, He Z, Daud MK, Li T, Yang X (2014) Impact assessment of cadmium toxicity and its bioavailability in human cell lines (Caco-2 and HL-7702). Biomed Res Int 2014:839538. https://doi.org/10.1155/2014/839538
Satpute RM, Hariharakrishnan J, Bhattacharya R (2008) Alpha-ketoglutarate and -acetyl cysteine protect PC12 cells from cyanide-induced cytotoxicity and altered energy metabolism. NeuroToxicology 29(1):170–178. https://doi.org/10.1016/0041-008X(87)90007-X
Brand RM, Cetin Y, Mueller C, Cuppett SL (2005) Effect of fatty acids on herbicide transport across Caco-2 cell monolayers. Toxicol in Vitro 19(5):595–601. https://doi.org/10.1016/j.tiv.2005.03.008
Liu K, Zheng J, Chen F (2018) Effects of washing, soaking, and domestic cooking on cadmium, arsenic, and lead bioaccessibilities in rice. J Sci Food Agric. https://doi.org/10.1007/s10653-018-0098-8
Minekus M, Alminger M, Alvito P, Balance S, Bohn T, Bourlieu C et al (2014) A standardised static in vitro digestion method suitable for food—an international consensus. Food Funct 5(6):1113–1124. https://doi.org/10.1039/c3fo60702j
Giorgi G, Roque ME (2014) Iron overload induces changes of pancreatic and duodenal divalent metal transporter 1 and prohepcidin expression in mice. Acta Histochem 116(2):354–362. https://doi.org/10.1016/j.acthis.2013.08.013
Pamela U, Pabla A, Andrés E, Victoria T, Mena NP, Miguel A et al (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126(4):541–549. https://doi.org/10.1111/jnc.12244
Min KS, Ueda H, Kihara T, Tanaka K (2008) Increased hepatic accumulation of ingested Cd is associated with upregulation of several intestinal transporters in mice fed diets deficient in essential metals. Toxicol Sci 106(1):284–289. https://doi.org/10.1093/toxsci/kfn14
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274(32):22739–22746. https://doi.org/10.1074/jbc.274.32.22739
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388(6641):482–488. https://doi.org/10.1038/41343
Funding
The authors would like to thank the financial support from the National Natural Science Foundation of China (No. 31771981) and Natural Science Foundation of Hunan Province of China under Grant (No. 2018JJ3877).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lv, Q., He, Q., Wu, Y. et al. Investigating the Bioaccessibility and Bioavailability of Cadmium in a Cooked Rice Food Matrix by Using an 11-Day Rapid Caco-2/HT-29 Co-culture Cell Model Combined with an In Vitro Digestion Model. Biol Trace Elem Res 190, 336–348 (2019). https://doi.org/10.1007/s12011-018-1554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1554-0