Abstract
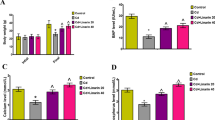
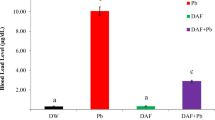
The purpose of this study was to evaluate the protective effect of Du-Zhong cortex extract (DZCE) on lead acetate-induced bone loss in rats. Forty female Sprague-Dawley rats were randomly divided into four groups: group I (control) was provided with distilled water. Group II (PbAc) received 500 ppm lead acetate in drinking water for 60 days. Group III (PbAc+DZCE) received 500 ppm lead acetate in drinking water, and given intragastric DZCE (100 mg/kg body weight) for 60 days. Group IV (DZCE) was given intragastric DZCE (100 mg/kg body weight) for 60 days. The bone mineral density, serum biochemical markers, bone histomorphology, and bone marrow adipocyte parameters were analyzed using dual-energy X-ray absorptiometry, biochemistry, histomorphometry, and histopathology, respectively. The results showed that the lumbar spine and femur bone mineral density was significantly decreased in PbAc group compared with the control (P < 0.05); however, this decrease was inhibited by the intake of Du-Zhong cortex extract (P < 0.05, vs. PbAc group; P > 0.05, vs. control and DZCE group). Serum calcium and serum phosphorus in the PbAc+DZCE group were greater than that in the PbAc group (P < 0.05). The PbAc group had higher ALP, osteocalcin, and RANKL than the control group (P < 0.01), and they were significantly lower in the PbAc+DZCE group compared with the PbAc group. There were no significant differences of ALP, osteocalcin, and RANKL among the PbAc+DZCE, control, and DZCE groups (P > 0.05). Serum OPG and OPG/RANKL ration were significantly higher in the PbAc+DZCE group than that in the PbAc group (P < 0.05). The bone histomorphometric analyses showed that bone volume and trabecular thickness in the femoral trabecular bone were significantly lower in the PbAc group than that in the control group, but those were restored in the PbAc+DZCE groups. The bone marrow adipocyte number, percent adipocyte volume per tissue volume (AV/TV), and mean adipocyte diameter were significantly increased in the PbAc group compared to the control (P < 0.01), and those were restored in the PbAc+DZCE group. The differences of those parameters between PbAc+DZCE, DZCE, and the control group were not significant. The results above indicate that the Du-Zhong cortex extract has protective effects on both stimulation of bone formation and suppression of bone resorption in lead-exposed rats, therefore, Du-Zhong cortex extract has the potential to prevent or treat osteoporosis resulting from lead expose.




Similar content being viewed by others
References
Conti M, Terrizzi A, Lee C et al (2012) Effects of lead exposure on growth and bone biology in growing rats exposed to simulated high altitude. Bull Environ Contam Toxicol 88(6):1033–1037
Monir AU, Gundberg CM, Yagerman SE, van der Meulen MCH, Budell WC, Boskey AL, Dowd TL (2010) The effect of lead on bone mineral properties from female adult C57/BL6 mice. Bone 47:888–894
Keson T, Thomas AG, Karen BR et al (2008) Associations of bone mineral density and lead levels in blood, tibia, and patella in urban-dwelling women. Environ Health Perspect 116(6):784–790
Escribano A, Revilla M, Hernández ER, Seco C, González-Riola J, Villa LF, Rico H (1997) Effect of lead on bone development and bone mass: a morphometric, densitometric, and histomorphometric study in growing rats. Calcified Tissue Int 60(2):200–203
Potula V, Kleinbaum D, Kaye W (2006) Lead exposure and spine bone mineral density. J Occup Environ Med 48:556–564
Chen X, Wang K, Wang Z, Gan C, He P, Liang Y, Jin T, Zhu G (2014) Effects of lead and cadmium coexposure on bone mineral density in a Chinese population. Bone 63:76–80
Campbell JR, Rosier RN, Novotny L, Puzas JE (2004) The association between environmental lead exposure and bone density in children. Environ Health Perspect 112:1200–1203
Campbell JR, Auinger P (2007) The association between blood lead levels and osteoporosis among adults—results from the third National Health and Nutrition Examination Survey (NHANES III). Environ Health Perspect 115:1018–1022
Beier EE, Maher JR, Sheu TJ, Cory-Slechta DA, Berger AJ, Zuscik MJ, Puzas JE (2013) Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling. Environ Health Perspect 121(1):97–104
Conti MI, Bozzini C, Facorro G et al (2012) Lead bone toxicity in growing rats exposed to chronic intermittent hypoxia. Bull Environ Contam Toxicol 89:693–698
Zhang R, Liu ZG, Li C, Hu SJ, Liu L, Wang JP, Mei QB (2009) Du-Zhong (Eucommia ulmoides Oliv.) cortex extract prevent OVX-induced osteoporosis in rats. Bone 45(3):553–559
Kim B, Lim DW, Song J et al (2012) Anti-osteoporotic effect of Eucommia ulmoides cortex in ovariectomized rats. Planta Med 78(11):1130–1130
Li HW, Deng JG, Du ZC et al (2013) Protective effects of mangiferin in subchronic developmental lead-exposed rats. Biol Trace Elem Res 152:233–242
Nagaraja H, Tan J, Srikumar C et al (2010) Protective effects of Etlingera elatior extract on lead acetate-induced changes in oxidative biomarkers in bone marrow of rats. Food and Chem Toxicol 48:2688–2694
Qi SS, Zheng HX (2017) Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res 177(2):281–285
Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S (2008) Effects of estrogen therapy on bone marrow adipocytes in post-menopausal osteoporotic women. Osteoporos Int 19:1323–1330
Li GW, Xu Z, Chang SX (2014) Influence of early zoledronic acid administration on bone marrow fat in ovariectomized rats. Endocrinology 155(12):4731–4738
Raafat BM, Hassan NS, Aziz SW (2012) Bone mineral density (BMD) and osteoporosis risk factor in Egyptian male and female battery manufacturing workers. Toxicol Ind Health 28(3):245–249
Nilima N, Dongre AN, Suryakar AJ et al (2013) Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Ind J Clin Biochem 28(1):65–70
Bassem MR, Nahed SH (2012) Bone mineral density (BMD) and osteoporosis risk factor in Egyptian male and female battery manufacturing workers. Toxicol Ind Health 28(3):245–252
Lee BK, Kim Y (2012) Association between bone mineral density and blood lead level in menopausal women: analysis of 2008–2009 Korean national health and nutrition examination. Environ Res 115(5):59–65
Pollack AZ, Mumford SL, Wactawski-Wende J, Yeung E, Mendola P, Mattison DR, Schisterman EF (2013) Bone mineral density and blood metals in premenopausal women. Environ Res 120(1):76–81
Gade TP, Motley MW, Beattie BJ, Bhakta R, Boskey AL, Koutcher JA, Mayer-Kuckuk P (2011) Imaging of alkaline phosphatase activity in bone tissue. PLoS One 6(7):e22608
Arimandi BH, Birnbaum RS, Juma S et al (2000) The synthetic phytoestrogen, ipriflavone, and estrogen prevent bone loss by different mechanisms. Calcified Tissue Int 66:61–65
Hamilton JD, OpFlaherty EJ (1999) Influence of lead on mineralization during bone growth. Fundam Appl Toxicol 26(2):265–271
Enjuanes A, Ruiz-Gaspà S, Peris P et al (2010) The effect of the alendronate on OPG/RANKL system in differentiated primary human osteoblasts. Endocrine 37(2):322–328
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 15(2):139–146
Hao Y, Gao R, Lu B, Ran Y, Yang Z, Liu J, Li R (2018) Ghrelin protects against depleted uranium-induced bone damage by increasing osteoprotegerin/RANKL ratio. Toxicol Appl Pharm 343(15):62–70
Liu JM, Zhao HY, Ning G, Zhao YJ, Chen Y, Zhang Z, Sun LH, Xu MY, Chen JL (2005) Relationships between the changes of serum levels of OPG and RANKL with age, menopause, bone biochemical markers and bone mineral density in Chinese women aged 20-75. Calcified Tissue Int 76(1):1–6
Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML (2010) Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res 25(9):2078–2088
Pino AM, Miranda M, Figueroa C et al (2016) Qualitative aspects of bone marrow adiposity in osteoporosis. Front Endocrinol 7(3):1–6
Maddalozzo GF, Turner RT, Edwards CH et al (2009) Alcohol alters whole body composition, inhibits bone formation, an increases bone marrow adiposity in rats. Osteoporos Int 20(9):1529–1538
Botolin S, Mccabe LR (2007) Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 148(1):198–205
Cornish JL, Wang T, Lin JM (2018) Role of marrow adipocytes in regulation of energy metabolism and bone homeostasis. Curr Osteoporos Rep 16(2):116–122
Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, Müller R, Kohler T, Zwahlen A, Lappe JM, Young P, Recker RR, Shane E (2012) Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 97(8):2782–2791
Yang Y, Luo X, Yan F, Jiang Z, Li Y, Fang C, Shen J (2015) Effect of zoledronic acid on vertebral marrow adiposity in postmenopausal osteoporosis assessed by MR spectroscopy. Skelet Radiol 44(10):1499–1505
Li BH, Liu H, Jia SN (2015) Zinc enhances bone metabolism in ovariectomized rats and exerts anabolic osteoblastic/adipocytic marrow effects ex vivo. Biol Trace Elem Res 163(1):202–207
Funding
This work was supported by the High-end Foreign Expert Recruitment Programme of State Administration of Foreign Experts Affairs (GDT20176100048), Qinling-Bashan Mountains Bioresources Comprehensive Development, Collaborative Innovation Center Research Funds QBXT-Z(P)-15-25, Shaanxi Science and Technology Coordinating Innovation Engineering Program (2015KTTSSF01-03, 2015HBGC-18), the Key Project of Agricultural Science and Technology of Shaanxi Province (2017NY-082), Postdoctoral Program in Shaanxi University of Technology (SLGBH16-03), and Research Project of Shaanxi Provincial Education Department (17JK0153).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors certify that there is no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Qi, S., Zheng, H., Chen, C. et al. Du-Zhong (Eucommia ulmoides Oliv.) Cortex Extract Alleviates Lead Acetate-Induced Bone Loss in Rats. Biol Trace Elem Res 187, 172–180 (2019). https://doi.org/10.1007/s12011-018-1362-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1362-6