Abstract
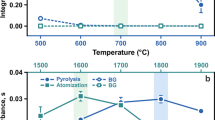
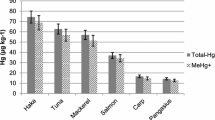
This paper presents a slurry sampling method for total mercury determination by graphite furnace atomic absorption spectrometry (GFAAS) in tissue of fish from the Amazon. The tissue samples were lyophilized and macerated, and then the slurry samples were prepared by putting 20 mg of tissue, added to a solution containing Triton X-100, Suprapur HNO3, and zirconium nitrate directly in sampling vials of a spectrometer. Mercury standard solutions were prepared under the same conditions as the slurry samples. The slurry samples and the mercury standard solutions were sonicated for 20 s. Twenty microliters of slurry samples were injected into the graphite tube, which contained an internal wall lined with tungsten carbide. Under these conditions, it was possible to thermally stabilize the mercury up to an atomization temperature of 1700 °C. The method was validated by mercury determination in reference materials DORM-4 and DOLT-4. The LOD and LOQ were 0.014 and 0.045 mg kg−1, respectively, and recovery percentages in relation to the concentration values were certified in the order of 98%.


Similar content being viewed by others
References
Moraes PM, Santos FA, Padilha CCF, Vieira JCS, Zara LF, de M. Padilha P (2012) A preliminary and qualitative metallomics study of mercury in the muscle of fish from Amazonas, Brazil. Biol Trace Elem Res 150(1-3):195–199. https://doi.org/10.1007/s12011-012-9502-x
Braga CP, Bittarello a C, Padilha CCF et al (2015) Mercury fractionation in dourada (Brachyplatystoma rousseauxii) of the Madeira River in Brazil using metalloproteomic strategies. Talanta 132:239–244. https://doi.org/10.1016/j.talanta.2014.09.021
Vieira JCS, Cavecci B, Queiroz JV, Braga CP, Padilha CCF, Leite AL, Figueiredo WS, Buzalaf MAR, Zara LF, Padilha PM (2015) Determination of the mercury fraction linked to protein of muscle and liver tissue of Tucunaré (Cichla spp.) from the Amazon region of Brazil. Arch Environ Contam Toxicol 69(4):422–430. https://doi.org/10.1007/s00244-015-0160-9
Vieira JCS, Braga CP, Oliveira G et al (2017) Identification of protein biomarkers of mercury toxicity in fish. Environ Chem Lett. https://doi.org/10.1007/s10311-017-0644-0
Cavalcante J, Vieira S, Braga CP, et al (2017) Mercury exposure: protein biomarkers of mercury exposure in Jaraqui fish from the Amazon region. Biol Trace Elem Res. https://doi.org/10.1007/s12011-017-1129-5
Moraes PM, Zamolin AP, Vargas AP, Golombieski RM, Loreto EL, Merritt TJS, Franco JL, Posser T (2012) Effects of Hg(II) exposure on MAPK phosphorylation and antioxidant system in D. melanogaster M. Environ Toxicol 29:621–630. https://doi.org/10.1002/tox.21788
Bełdowska M, Falkowska L (2016) Mercury in marine fish, mammals, seabirds, and human hair in the coastal zone of the southern Baltic. Water Air Soil Pollut 227(2):52. https://doi.org/10.1007/s11270-015-2735-5
Walters DM, Rosi-Marshall E, Kennedy TA, Cross WF, Baxter CV (2015) Mercury and selenium accumulation in the Colorado River food web, Grand Canyon, USA. Environ Toxicol Chem SETAC 34(10):2385–2394. https://doi.org/10.1002/etc.3077
Borrell A, Tornero V, Bhattacharjee D, Aguilar A (2015) Trace element accumulation and trophic relationships in aquatic organisms of the Sundarbans mangrove ecosystem (Bangladesh). Sci Total Environ 545–546:414–423. https://doi.org/10.1016/j.scitotenv.2015.12.046
Amoli JS, Barin A, Ebrahimi-Rad M, Sadighara P (2011) Cell damage through pentose phosphate pathway in fetus fibroblast cells exposed to methyl mercury. J Appl Toxicol 31(7):685–689. https://doi.org/10.1002/jat.1628
Belyaeva EA, Korotkov SM, Saris N-E (2011) In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: a comparison of copper and mercury with cadmium. J Trace Elem Med Biol 25(Suppl 1):S63–S73. https://doi.org/10.1016/j.jtemb.2010.10.007
Silva F, Padilha C, Pezzato L, Barros M, Padilha P (2006) Determination of chromium by GFAAS in slurries of fish feces to estimate the apparent digestibility of nutrients in feed used in pisciculture. Talanta 69(4):1025–1030. https://doi.org/10.1016/j.talanta.2005.12.008
Loureiro VR, Saleh MAD, Moraes PM, Neves RCF, Silva FA, Padilha CCF, Padilha PM (2007) Manganese determination by GFAAS in feces and fish feed slurries. J Braz Chem Soc 18(6):1235–1241. https://doi.org/10.1590/S0103-50532007000600019
Silva FA, Neves RCF, Quintero-Pinto LG, Padilha CCF, Jorge SMA, Barros MM, Pezzato LE, Padilha PM (2007) Determination of selenium by GFAAS in slurries of fish feces to estimate the bioavailability of this micronutrient in feed used in pisciculture. Chemosphere 68(8):1542–1547. https://doi.org/10.1016/j.chemosphere.2007.03.003
Schalm OW, Oscar W, Jain NC, Nemi C (1986) Schalm’s veterinary hematology, 4th edn. Lea & Febiger, Philadelphia
Moraes PM, Santos FA, Cavecci B, Padilha CCF, Vieira JCS, Roldan PS, Padilha PM (2013) GFAAS determination of mercury in muscle samples of fish from Amazon, Brazil. Food Chem 141(3):2614–2617. https://doi.org/10.1016/j.foodchem.2013.05.008
Currie RJW (1999) Ascites in poultry: recent investigations. Avian Pathol 28(4):313–326. https://doi.org/10.1080/03079459994560
FAO/WHO (2004) Human energy requirements. FAO Food and nutrition technical report series 1 0:6
Horwitz W, Albert R (1997) The concept of uncertainty as applied to chemical measurements. Analyst 122(6):615–617. https://doi.org/10.1039/a703178e
Bermejo-Barrera P, Moreda-Piñeiro J, Moreda-Piñeiro A, Bermejo-Barrera A (1994) Palladium as a chemical modifier for the determination of mercury in marine sediment slurries by electrothermal atomization atomic absorption spectrometry. Anal Chim Acta 296(2):181–193. https://doi.org/10.1016/0003-2670(94)80262-9
Bermejo-Barrera P, Verdura-Constenla EM, Moreda-Piñeiro A, Bermejo-Barrera A (1999) Rapid acid leaching and slurry sampling procedures for the determination of methyl-mercury and total mercury in human hair by electrothermal atomic absorption spectrometry. Anal Chim Acta 398(2-3):263–272. https://doi.org/10.1016/S0003-2670(99)00453-5
Moreda-Piñeiro J, López-Mahía P, Muniategui-Lorenzo S, Fernández-Fernández E, Prada-Rodríguez D (2002) Direct mercury determination in aqueous slurries of environmental and biological samples by cold vapour generation–electrothermal atomic absorption spectrometry. Anal Chim Acta 460(1):111–122. https://doi.org/10.1016/S0003-2670(02)00137-X
Zhang Y, Adeloju SB (2012) Speciation of mercury in fish samples by flow injection catalytic cold vapour atomic absorption spectrometry. Anal Chim Acta 721:22–27. https://doi.org/10.1016/j.aca.2012.01.038
Gallignani M, Bahsas H, Brunetto MR, Burguera M, Burguera JL, Petit de Peña Y (1998) A time-based flow injection-cold vapor-atomic absorption spectrometry system with on-line microwave sample pre-treatment for the determination of inorganic and total mercury in urine. Anal Chim Acta 369(1-2):57–67. https://doi.org/10.1016/S0003-2670(98)00217-7
Acknowledgements
The authors thank the Brazilian research funding agencies FAPESP (Processes: 2010/51332-5 and 2013/21297-1) and ANEEL/ESBR- P&D: 6631-0001/2012/Contract Jirau 004/201 for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the work in this paper that involved animal experimentation was approved by the Ethics Committee on the Use of Animals (CEUA) of the Faculty of Veterinary Medicine and Zootechnics (FMVZ) of the São Paulo State University (UNESP), School of Veterinary Medicine and Animal Science, Botucatu, Brazil, under the number of protocol 110/2015.
Rights and permissions
About this article
Cite this article
de Queiroz, J.V., Vieira, J.C.S., da Cunha Bataglioli, I. et al. Total Mercury Determination in Muscle and Liver Tissue Samples from Brazilian Amazon Fish Using Slurry Sampling. Biol Trace Elem Res 184, 517–522 (2018). https://doi.org/10.1007/s12011-017-1212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1212-y