Abstract
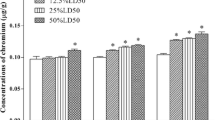
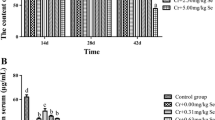
Selenium (Se) can play a protective role against heavy metal toxicity. This experiment aims to evaluate the effect of Se supplementation at different doses on the chicken brains. Oxidative stress was induced in the chicken brains by chromium(VI). A total of 105 Hyland brown male chickens were randomly divided into seven groups, including the control group, poisoned group [6%LD50 K2Cr2O7 body weight (B.W.)], and detoxification groups K2Cr2O7 (6%LD50) + Se (0.31, 0.63, 1.25, 2.50, and 5.00 Na2SeO3 mg/kg B.W.) orally in water for 42 days. The chickens were detected by the activities of mitochondrial membrane potential, 2′-benzoyloxycinnamaldehyde, superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), and Ca2+-ATPase. Cr(VI) administration caused histopathological damage. In addition, changes in oxidative stress indicators were observed in the chicken’s brains. Se supplement increased the levels of GSH, mitochondrial membrane potential (MMP), and Ca2+-ATPase and reduced MDA activity in the detoxification groups. However, the high-dose Se supplementation groups of 2.50 and 5.00 mg/kg reduced the activities of GSH, MMP, and Ca2+-ATPase; increased the brain–body ratio; and increased SOD activity. In conclusion, Cr(VI) exposure caused oxidative stress. Se exerted a remission effect on toxic responses in the chicken brains. However, a high Se concentration was synergistic to the toxic effect of Cr(VI).






Similar content being viewed by others
References
Lou J, Jin L, Wu N, Tan Y, Song Y, Gao M, Liu K, Zhang X, He J (2013) DNA damage and oxidative stress in human B lymphoblastoid cells after combined exposure to hexavalent chromium and nickel compounds. Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association 55C:533–540
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB (2009) Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol 24:66–73
Myers CR (2012) The effects of chromium(VI) on the thioredoxin system: implications for redox regulation. Free Radic Biol Med 52:2091–2107
Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: a double-edged sword. Chem Biol Interact 188:276–288
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Travacio M, JMA P, Llesuy S (2001) Erratum to “chromium (VI) induces oxidative stress in the mouse brain”: [toxicology 150 (2000) 137–146]. Toxicology 162:139–148
Yon JM, Baek IJ, Lee SR, Yan J, Kim MR, Nahm SS, Kim JS, Ahn B, Lee BJ, Yun YW (2008) The spatio-temporal expression pattern of cytoplasmic Cu/Zn superoxide dismutase (SOD1) mRNA during mouse embryogenesis. J Mol Histol 39:95–103
Kumar V, Gill KD (2014) Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: a review. Neurotoxicology 41:154–166
Strehler EE (2015) Plasma membrane calcium ATPases: from generic Ca 2+ sump pumps to versatile systems for fine-tuning cellular Ca 2+. Biochemical & Biophysical Research Communications 460:26–33
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268
JX X, Cao CY, Sun YC, Wang LL, Li N, SW X, Li JL (2014) Effects on liver hydrogen peroxide metabolism induced by dietary selenium deficiency or excess in chickens. Biol Trace Elem Res 159:174–182
Naziroğlu M, Çelik Ö, Uğuz AC, Bütün A (2015) Protective effects of riboflavin and selenium on brain microsomal Ca2 + −ATPase and oxidative damage caused by glyceryl trinitrate in a rat headache model. Biol Trace Elem Res 164:72–79
Prashant Babaji M, Bc Manjunath M, Mahesh Melkundi M, Rani S, Vatchala R (2011) Dietary selenium reduces retention of methyl mercury in freshwater fish. Environmental Science & Technology 45:9793–9798
Chen X, Zhu YH, Cheng XY, Zhang ZW, SW X (2012) The protection of selenium against cadmium-induced cytotoxicity via the heat shock protein pathway in chicken splenic lymphocytes. Molecules 17:14565–14572
Soudani N, Troudi A, Amara IB, Bouaziz H, Boudawara T, Zeghal N (2012) Ameliorating effect of selenium on chromium (VI)-induced oxidative damage in the brain of adult rats. Journal of Physiology & Biochemistry 68:397–409
Xu T, Gao X, Liu G (2016) The antagonistic effect of selenium on lead toxicity is related to the ion profile in chicken liver. Biol Trace Elem Res 169:1–9
Horn HJ (1956) Simplified LD 50 (or ED 50 ) calculations. Biometrics 12:311–322
Zhu FH, Zhu LQ, Li L, Sun JQ, Chen F (2010) Effects of hige levels of nano-Se on blood selenium content and antioxidant abilities in hens. Chinese Journal of Animal Science 46:31–34
Boyne AF, Ellman GL (1972) A methodology for analysis of tissue sulfhydryl components. Anal Biochem 46:639–653
Bagchi D, Hassoun EA, Bagchi M, Stohs SJ (1995) Chromium-induced excretion of urinary lipid metabolites, DNA damage, nitric oxide production, and generation of reactive oxygen species in Sprague-Dawley rats. Comparative biochemistry and physiology Part C, Pharmacology, toxicology & endocrinology 110:177–187
Kadiiska MB, Xiang QH, Mason RP (1994) In vivo free radical generation by chromium(VI): an electron spin resonance spin-trapping investigation. Chem Res Toxicol 7:800–805
Zwolak I, Zaporowska H (2011) Selenium interactions and toxicity: a review. Selenium interactions and toxicity. Cell Biology & Toxicology 28:31–46
Sugiyama M (1992) Role of physiological antioxidants in chromium(VI)-induced cellular injury. Free Radic Biol Med 12:397–407
Kart A, Koc E, Dalginli KY, Gulmez C, Sertcelik M, Atakisi O (2016) The therapeutic role of glutathione in oxidative stress and oxidative DNA damage caused by hexavalent chromium. Biological Trace Element Research:1–5
Bagchi D, Bagchi M, Stohs SJ (2001) Chromium (vi)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor Gene. Molecular & Cellular Biochemistry 222:149–158
Soudani N, Amara IB, Sefi M, Boudawara T, Zeghal N (2011) Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Experimental & Toxicologic Pathology Official Journal of the Gesellschaft Fur Toxikologische Pathologie 63:541–548
Liu L, Yang B, Cheng Y, Lin H (2015) Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biol Trace Elem Res 167:1–12
Li X, Hill KE, Burk RF, May JM (2001) Selenium spares ascorbate and K-tocopherol incultured liver cell lines under oxidant stress. FEBS Lett 508:489–492
Wang HW, Wang JX, Yang LK, Liu L, Lu SS, Yang FK, Cai DB (2014) Effects of dietary selenium supplements on the superoxide dismutase (SOD) activity of Neocaridina heteropoda (Crustacea: Decapoda: Atyidae: Caridina ) exposed to ambient sodium polyphosphate. Adv Mater Res 1073-1076:1841–1843
Travacio M, Llesuy S (1996) Antioxidant enzymes and their modification under oxidative stress conditions. Ciênccult 48:9–13
Kouba A, Velíšek J, Stará A, Masojídek J, Kozák P (2014) Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus). Biomed Res Int 2014:143–146
Tuzen M, Pekiner OZ (2015) Ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction combined with graphite furnace atomic absorption spectrometric for selenium speciation in foods and beverages. Food Chem 188:619–624
Spallholz JE, Hoffman DJ (2002) Selenium toxicity: cause and effects in aquatic birds. Aquat Toxicol 57:27–37
Silva MAOD, Andrade SALD, Mazzafera P, Arruda MAZ (2011) Evaluation of sunflower metabolism from zinc and selenium addition to the culture: a comparative metallomic study. Int J Mass Spectrom 307:55–60
Amado LL, Monserrat JM (2010) Oxidative stress generation by microcystins in aquatic animals: why and how. Environ Int 36:226–235
Bütün A, Nazıroğlu M, Demirci S, Celik O, Uğuz AC (2015) Riboflavin and vitamin E increase brain calcium and antioxidants, and microsomal calcium-ATP-ase values in rat headache models induced by glyceryl trinitrate. J Membr Biol 248:205–213
Naziroğlu M, Kutluhan S, Yilmaz M (2008) Selenium and topiramate modulates brain microsomal oxidative stress values, Ca2 + −ATPase activity, and EEG records in pentylentetrazol-induced seizures in rats. J Membr Biol 225:39–49
Rossi SC, Gorman N, Wetterhahn KE (1988) Mitochondrial reduction of the carcinogen chromate: formation of chromium(V). Chem Res Toxicol 1(2):101–107
Khan FH, Ambreen K, Fatima G, Kumar S (2012) Assessment of health risks with reference to oxidative stress and DNA damage in chromium exposed population. Sci Total Environ 430:68–74
Travacio M, Polo JMA, Llesuy S (2000) Chromium(VI) induces oxidative stress in the mouse brain. Toxicology 150:137–146
Simoni J, Feola M Selenium protection from cadmium and chromium poisoning. In, 1986. pp 371–371
Battin EE, Zimmerman MT, Ramoutar RR, Quarles CE, Brumaghim JL (2011) Preventing metal-mediated oxidative DNA damage with selenium compounds. Metallomics 3:503–512
Acknowledgements
This work was supported by the National Key R&D Program (2016YFD0501208), the Shandong Modern Agricultural Technology & Industry System (No. SDAIT-11-04).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Pan Hao and Yiran Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hao, P., Zhu, Y., Wang, S. et al. Selenium Administration Alleviates Toxicity of Chromium(VI) in the Chicken Brain. Biol Trace Elem Res 178, 127–135 (2017). https://doi.org/10.1007/s12011-016-0915-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0915-9