Abstract
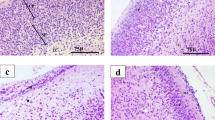
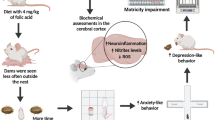
Methyl mercury (MeHg) is a developmental neurotoxin that causes irreversible cognitive damage in offspring of gestationally exposed mothers. Currently, no preventive drugs are established against MeHg developmental neurotoxicity. The neuroprotective effect of gestational administration of a flavanoid against in utero toxicity of MeHg is not explored much. Hence, the present study validated the effect of a bioactive flavanoid, fisetin, on MeHg developmental neurotoxicity outcomes in rat offspring at postnatal weaning age. Pregnant Wistar rats were simultaneously given MeHg (1.5 mg/kg b.w.) and two doses of fisetin (10 and 50 mg/kg b.w. in two separate groups) orally from gestational day (GD) 5 till parturition. Accordingly, after parturition, on postnatal day (PND) 24, weaning F1 generation rats were studied for motor and cognitive behavioural changes. Biochemical and histopathological changes were also studied in the cerebral cortex, cerebellum and hippocampus on PND 25. Administration of fisetin during pregnancy prevented behavioural impairment due to transplacental MeHg exposure in weaning rats. Fisetin decreased the levels of oxidative stress markers, increased enzymatic and non-enzymatic antioxidant levels and increased the activity of membrane-bound ATPases and cholinergic function in F1 generation rats. In light microscopic studies, fisetin treatment protected the specific offspring brain regions from significant morphological aberrations. Between the two doses of fisetin studied, 10 mg/kg b.w. was found to be more satisfactory and effective than 50 mg/kg b.w. The present study shows that intake of fisetin during pregnancy in rats ameliorated in utero MeHg exposure-induced neurotoxicity outcomes in postnatal weaning F1 generation rats.















Similar content being viewed by others
References
Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA (2014) Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92:254–269
Gary J, Myers PW, Davidson WGE, Van EW, Thurston SW, Strain JJ, Shamlaye CF, Bovetd P (2014) Methylmercury exposure and developmental neurotoxicity. Bull World Health Organ 93(2):132
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Johnson CL (2004) Mercury in the environment: sources, toxicities, and prevention of exposure. Pediatr Ann 33:437–442
Matsumoto H, Koya G, Takeuchi T (1965) Fetal Minamata disease. A neuropathological study of two cases of intrauterine intoxication by a methyl mercury compound. J Neuropathol Exp Neurol 24:563–574
Choi BH, Lapham LW, Amin-Zaki L, Saleem T (1978) Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol 37:719–733
Takeuchi T (1982) Pathology of Minamata disease. With special reference to its pathogenesis. Acta Pathol Jpn 32(Suppl 1):73–99
Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, Choi A, Cernichiari E, Choisy O, Clarkson TW (1995) Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology 16:653–664
Van WE, Thurston SW, Myers GJ, Strain JJ, Weiss B, Zarcone T, Watson GE, Zareba G, McSorley EM, Mulhern MS, Yeates AJ, Henderson J, Gedeon J, Shamlaye CF, Davidson PW (2013) Prenatal methyl mercury exposure in relation to neurodevelopment and behavior at 19 years of age in the Seychelles Child Development Study. Neurotoxicol Teratol 39:19–25
Aschner M, Syversen T, Souza DO, Rocha JB, Farina M (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40:285–291
Halliwell B (1992) Oxygen radicals as key mediators in neurological disease: fact or fiction? Ann Neurol 32:10–15
Mori N, Yasutake A, Hirayama K (2007) Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol 81:769–776
Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M (2007) Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol 20:1919–1926
Mari M, Morales M, Colell A, García-Ruiz C, Jose C, Fernández-Checa (2009) Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 11:2685–2700
Farina M, Rocha JBT, Aschner M (2011) Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89:555–563
Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M (2008) Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227:147–154
Mynett-Johnson L, Murphy V, McCormack J, Shields DC, Claffey E, Manley P, McKeon P (1998) Evidence for an allelic association between bipolar disorder and Na+, K+ adenosine triphosphatase alpha subunit gene (ATP1A3). Biol Psychiatry 44:47–51
Wood AJ, Elphick M, Grahame-Smith DG (1989) Effect of lithium and of other drugs used in the treatment of manic illness on the cation-transporting properties of Na+, K+-ATPase in mouse brain synaptosomes. J Neurochem 52:1042–1049
Erecinska M, Silver IA (1994) Ions and energy in mammalian brain. Prog Neurobiol 43:37–71
Carageorgiou H, Pantos C, Zarros A, Stolakis V, Mourouzis I, Cokkinos D, Tsakiris S (2007) Changes in acetylcholinesterase, Na+K+ ATPase, and Mg2+ ATPase activities in the frontal cortex and the hippocampus of hyper and hypothyroid adult rats. Metab Clin Exp 56:1104–1110
Chiu VC, Mouring D, Haynes DH (1983) Action of mercurials on the active and passive transport properties of sarcoplasmic reticulum. J Bioenerg Biomembr 15:13–25
Freitas AJ, Rocha JB, Wolosker H, Souza DO (1996) Effects of Hg2+ and CH3Hg+ on Ca2+ fluxes in rat brain microsomes. Brain Res 738:257–264
Haynes DH (1983) Mechanism of Ca2+ transport by Ca2+-Mg2+-ATPase pump: analysis of major states and pathways. Am J Phys 244:G3–12
Everitt BJ, Robbins TW (1997) Central cholinergic systems and cognition. Annu Rev Psychol 48:649–684
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97:320–324
Appleyard ME (1995) Acetylcholinesterase induced long-term potentiation in CA1 pyramidal cells by a mechanism dependent on metabotropic glutamate receptors. Neurosci Lett 190:25–28
Petruccioli L, Turillazzi P (1991) Effect of methylmercury on acetylcholinesterase and serum cholinesterase activity in monkeys, Macaca fascicularis. Bull Environ Contam Toxicol 46:769–773
Wootten V, Brown DR, Callahan BG, Vetrano K, Wadman P, Melia J, Mulligan T, Schatz RA (1985) Behavioral and biochemical alterations following in utero exposure to methylmercury. Neurobehav Toxicol Teratol 7:767–773
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2:191–206
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34
Deeba N, Syed Vaqar M, Adhami M, Khan I, Mukhtar H (2013) Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anti Cancer Agents Med Chem 13:995–1001
Zhen L, Zhu J, Zhao X, Wu H, An Y, Li S, Du X, Lin M, Wang Q, Xu Y, Pan J (2012) The antidepressant like effect of fisetin involves the serotonergic and noradrenergic system. Behav Brain Res 228:359–366
Amin-Zaki L, Majeed MA, Elhassani SB, Clarkson TW, Greenwood MR, Doherty RA (1979) Prenatal methylmercury poisoning. Clinical observations over five years. Am J Dis Child 133:172–177
Khan N, Deeba N, Syed MH (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19:151–162
Chuang JY, Chang PC, Shen YC et al (2014) Regulatory effects of fisetin on microglial activation. Molecules 15:8820–8839
Prakash D, Gopinath K, Sudhandiran G (2013) Fisetin enhances behavioral performances and attenuates reactive gliosis and inflammation during aluminum chloride-induced neurotoxicity. NeuroMolecular Med 15:192–208
Glowinski J, Iversen LL (1996) Regional studies of catecholamines in the rat brain. J Neurochem 13:655–669
Sakamoto M, Nakano A, Kajiwara Y, Naruse I, Fujisaki T (1993) Effects of methyl mercury in postnatal developing rats. Environ Res 61:43–50
Onishchenko N, Tamm C, Vahter M, Tomas H, Johnson JA, DA J, Sandra C (2007) Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicological Sci 97:428–437
Yoshida M, Suzuki M, Satoh M, Yasutake A, Watanabe C (2011) Neurobehavioral effects of combined prenatal exposure to low-level mercury vapor and methylmercury. J Toxicol Sci 36:73–80
Beyrouty P, Stamler CJ, Liu JN, Loua KM, Kubow S, Chan HM (2006) Effects of prenatal methylmercury exposure on brain monoamine oxidase activity and neurobehaviour of rats. Neurotoxicol Teratol 28:251–259
Lowry OH, Risebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–270
Devasagayam TP, Tarachand U (1987) Decreased lipid peroxidation in rat kidneys during gestation. Biochem Biophys Res Commun 145:134–138
Levine RL, Williams JA, Stadtman ER, Shater E (1994) Carbonyl, assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–152
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bonting SL (1970) Sodium-potassium activated adenosinetriphosphatase and cation transport. In Bittar EE (ed) Membranes and ion transport. Interscience Publishers, Ltd., London, 1:257–263
Hjerton S, Pan H (1983) Purification and characterization of two forms of a low affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta 728:281–288
Ohnishi T, Suzuki T, Suzuki Y, Ozawa K (1982) A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta 684:67–74
Fiske CK, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:376–406
Worek F, Eyer P, Thiermann H (2012) Determination of acetylcholinesterase activity by the Ellman assay: a versatile tool for in vitro research on medical countermeasures against organophosphate poisoning. Drug Test Anal 4:282–291
Feldman AT, Wolfe D (2014) Tissue processing and hematoxylin and eosin staining. Methods Mol Biol 1180:31–43
Furuta Y, Kobori O, Shimazu H, Morioka Y, Okuyama Y (1985) A new in vivo staining method, cresyl violet staining, for fiberoptic magnified observation of carcinoma of the gastric mucosa. Gastroenterol Jpn 20:120–124
Nielsen JB, Andersen O (1992) The toxicokinetics of mercury in mice offspring after maternal exposure to methylmercury—effect of selenomethionine. Toxicology 74:233–241
Mansour MM, Dyer NC, Hoffman LH, Schulert AR, Brill AB (1973) Maternal-fetal transfer of organic and inorganic mercury via placenta and milk. Environ Res 6:479–484
Kerper LE, Ballatori N, Clarkson TW (1992) Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Phys 26:761–765
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Exp Biol Med 222:236–245
Yaginuma-Sakurai K, Murata K, Iwai-Shimada M, Nakai K, Kurokawa N, Tatsuta N, Satoh H (2012) Hair-to-blood ratio and biological half-life of mercury: experimental study of methylmercury exposure through fish consumption in humans. J Toxicol Sci 37:123–130
Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia PSC, Moro AM, Bohrer D, Bairros AV, Dafre AL, Santos AR, Farina M (2006) Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res 102:22–28
Gimenez-Llort L, Ahlbom E, Dare E, Vahter M, Ogren S, Ceccatelli S (2001) Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol 9:61–70
Maher P, Dargusch R, Bodai L, Paul E, Gerard PJM, Marsh JL (2011) ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet 20:261–270
Wu J, Cheng G, Lu Z, Wang M, Tian J, Bi Y (2016) Effects of methyl mercury chloride on rat hippocampus structure. Biol Trace Elem Res 171:124–130
Ravichandran N, Suresh G, Ramesh B, Siva GV (2011) Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem Toxicol 49(5)
Kanda H, Shinkai Y, Kumagai Y (2014) S-Mercuration of cellular proteins by methylmercury and its toxicological implications. J Toxicol Sci 39:687–700
Chiruta C, Schubert D, Dargusch R, Maher P (2012) Chemical modification of the multitarget neuroprotective compound fisetin. J MedChem 55:378–389
Magour S (1986) Studies on the inhibition of brain synaptosomal Na+/K+-ATPase by mercury chloride and methyl mercury chloride. Arch Toxicol 9:393–396
Chuu JJ, Liu SH, Lin-Shiau SY (2001) Effects of methyl mercury, mercuric sulfide and cinnabar on active avoidance responses, Na+/K+-ATPase activities and tissue mercury contents in rats. Proc. Natl. Sci. Counc. Repub. China B 25:128–136
Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb 3:1–30
Zhang Y, Lu R, Liu W, Wu Y, Qian H, Zhao X, Wang S, Xing G, Yu F, Aschner M (2013) Hormetic effects of acute methylmercury exposure on grp78 expression in rat brain cortex. Dose Response 11:109–120
Cheng JP, Yang YC, Hu WX, Yang L, Wang WH, Jia JP, Lin XY (2005) Effect of methylmercury on some neurotransmitters and oxidative damage of rats. J Environ Sci 17:469–473
Gao Y, Yan CH, Yu XD, Wu SH (2006) Effects of perinatal exposure to methylmercury on the structure of hippocampus and cerebellum in young rats. Wei Sheng Yan Jiu 35:402–405
Falluel-More A, Sokolowski K, Sisti HM, Zhou X, Tracey J, Shors TJ, DiCicco-Bloom E (2007) Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem 103:1968–1981
Burbacher TM, Rodier PM, Weiss B (1990) Methylmercury developmental neurotoxicity: a comparison of effects in humans and animals. Neurotoxicol Teratol 12:191–202
Carvalho MC, Nazari EM, Farina M, Muller YMR (2008) Behavioral, morphological, and biochemical changes after in ovo exposure to methylmercury in chicks. Toxicol Sci 106:180–185
Acknowledgments
The financial assistance and award of Junior Research Fellowship (JRF) to the first author under the Department of Science and Technology - Promotion of University Research and Scientific Excellence (DST - PURSE) Phase II programme is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jacob, S., Thangarajan, S. Effect of Gestational Intake of Fisetin (3,3′,4′,7-Tetrahydroxyflavone) on Developmental Methyl Mercury Neurotoxicity in F1 Generation Rats. Biol Trace Elem Res 177, 297–315 (2017). https://doi.org/10.1007/s12011-016-0886-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0886-x