Abstract
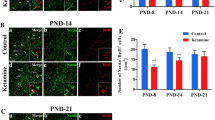
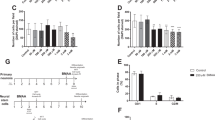
Hippocampal neurogenesis-related structural damage, particularly that leading to defective adult cognitive function, is considered an important risk factor for neurodegenerative and psychiatric diseases. Normal differentiation of neurons and glial cells during development is crucial in neurogenesis, which is particularly sensitive to the environmental toxicant methylmercury (MeHg). However, the exact effects of MeHg on hippocampal neural stem cell (hNSC) differentiation during puberty remain unknown. This study investigates whether MeHg exposure induces changes in hippocampal neurogenesis and whether these changes underlie cognitive defects in puberty. A rat model of methylmercury chloride (MeHgCl) exposure (0.4 mg/kg/day, PND 5–PND 33, 28 days) was established, and the Morris water maze was used to assess cognitive function. Primary hNSCs from hippocampal tissues of E16-day Sprague–Dawley rats were purified, identified, and cloned. hNSC proliferation and differentiation and the growth and morphology of newly generated neurons were observed by MTT and immunofluorescence assays. MeHg exposure induced defects in spatial learning and memory accompanied by a decrease in number of doublecortin (DCX)-positive cells in the dentate gyrus (DG). DCX is a surrogate marker for newly generated neurons. Proliferation and differentiation of hNSCs significantly decreased in the MeHg-treated groups. MeHg attenuated microtubule-associated protein-2 (MAP-2) expression in neurons and enhanced the glial fibrillary acidic protein (GFAP)-positive cell differentiation of hNSCs, thereby inducing degenerative changes in a dose-dependent manner. Moreover, MeHg induced deficits in hippocampus-dependent spatial learning and memory during adolescence as a consequence of decreased generation of DG neurons. Our findings suggested that MeHg exposure could be a potential risk factor for psychiatric and neurodegenerative diseases.







Similar content being viewed by others
References
Drapeau E, Mayo W, Aurousseau C, Le MM, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A 100(24):14385–14390
Kaneko N, Sawamoto K (2009) Adult neurogenesis and its alteration under pathological conditions. Neurosci Res 63(3):155–164
Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11(8):901–907
Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cinà I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F (2008) The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS Biol 6(10):e246
Garthe A, Behr J, Kempermann G (2009) Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 4(5):e5464
Dupret D, Revest JM, Koehl M, Ichas F, De GF, Costet P, Abrous DN, Piazza PV (2008) Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3(4):e1959
Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5(8):e214
Clelland CD, Choi M, Romberg C, Clemenson Jr GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325(5937):210–213
Ransome MI, Renoir T, Hannan AJ (2012) Hippocampal neurogenesis, cognitive deficits and affective disorder in Huntington’s disease. Neural Plast 2012:874387
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281
Llorens-Martín M, Blazquez-Llorca L, Benavides-Piccione R, Rabano A, Hernandez F, Avila J, DeFelipe J (2014) Selective alterations of neurons and circuits related to early memory loss in Alzheimer’s disease. Front Neuroanat 8:38
Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J (2014) Ezh2 regulates adult hippocampal neurogenesis and memory. J Neurosci. 34(15):5184–5199
Hodge RD, Nelson BR, Kahoud RJ, Yang R, Mussar KE, Reiner SL, Hevner RF (2012) Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci. 32(18):6275–6278
Wang J, Cheng A, Wakade C, Yu RK (2014) Ganglioside GD3 is required for neurogenesis and long-term maintenance of neural stem cells in the postnatal mouse brain. J Neurosci. 34(41):13790–13800
Ekthuwapranee K, Sotthibundhu A, Tocharus C, Govitrapong P (2015) Melatonin ameliorates dexamethasone-induced inhibitory effects on the proliferation of cultured progenitor cells obtained from adult rathippocampus. J Steroid Biochem Mol Biol 145:28–38
Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94(4):991–1026
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27(8):447–452
Ihrie RA, Alvarez-Buylla A (2008) Cells in the astroglial lineage are neural stem cells. Cell Tissue Res 331(1):179–191
Bernal GM, Peterson DA (2011) Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell 10(3):466–482
Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G (2006) Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54(8):805–814
Corty MM, Freeman MR (2013) Cell biology in neuroscience: architects in neural circuit design: glia control neuron numbers and connectivity. J Cell Biol 203(3):395–405
Cameron HA, Mckay RD (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435(4):406–417
Taylor CJ, Jhaveri DJ, Bartlett PF (2013) The therapeutic potential of endogenous hippocampal stem cells for the treatment of neurological disorders. Front Cell Neurosci 7:5
Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang MH (2012) Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012:854285
Kee N, Teixeira CM, Wang AH, Frankland PW (2007) Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci 10(3):355–362
Deng W, Saxe MD, Gallina IS, Gage FH (2009) Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 29(43):13532–13542
Tashiro A, Makino H, Gage FH (2007) Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 27(12):3252–3259
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85
Rodríguez JJ, Verkhratsky A (2011) Neurogenesis in Alzheimer’s disease. J Anat 219(1):78–89
Abdel-Salam OM (2011) Stem cell therapy for Alzheimer’s disease. CNS Neurol Disord Drug Targets 10(4):459–485
Samuels BA, Hen R (2011) Neurogenesis and affective disorders. Eur J Neurosci. 33(6):1152–1159
Danzer SC (2012) Depression, stress, epilepsy and adult neurogenesis. Exp Neurol 233(1):22–32
Burke K, Cheng Y, Li B, Petrov A, Joshi P, Berman RF, Reuhl KR, DiCicco-Bloom E (2006) Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology 27(6):970–981
Decimo I, Bifari F, Krampera M, Fumagalli G (2012) Neural stem cell niches in health and diseases. Curr Pharm Des 18(13):1755–1783
Snyder JS, Hong NS, Mcdonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130(4):843–852
Neal RE, Chen J, Jagadapillai R, Jang H, Abomoelak B, Brock G, Greene RM, Pisano MM (2014) Developmental cigarette smoke exposure: hippocampus proteome and metabolome profiles in low birth weight pups. Toxicology 317:40–49
Wu CC, Hung CJ, Shen CH, Chen WY, Chang CY, Pan HC, Liao SL, Chen CJ (2014) Prenatal buprenorphine exposure decreases neurogenesisin rats. Toxicol Lett 225(1):92–101
Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S (2011) Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 33(10):1833–1840
Clarkson TW, Vyas JB, Ballatori N (2007) Mechanisms of mercury disposition in the body. Am J Ind Med 50(10):757–764
Tamm C, Duckworth J, Hermanson O, Ceccatelli S (2006) High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem 97(1):69–78
Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E (2007) Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem 103(5):1968–1981
Haase H, Engelhardt G, Hebel S, Rink L (2011) Mercuric ions inhibit mitogen-activated protein kinase dephosphorylation by inducing reactive oxygen species. Toxicol Aplli Pharmacol 250(1):78–86
Sokolowski K, Falluel-Morel A, Zhou X, DiCicco-Bloom E (2011) Methylmercury (MeHg) elicits mitochondrial-dependent apoptosis in developing hippocampus and acts at low exposures. Neurotoxicology 32(5):535–544
Sokolowski K, Obiorah M, Robinson K, McCandlish E, Buckley B, Di Cicco-Bloom E (2013) Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits. Dev Neurobiol. 73(12):936–949
Georg Kuhn H, Blomgren K (2011) Developmental dysregulation of adult neurogenesis. Eur J Neurosci. 33(6):1115–1122
Onishchenko N, Tamm C, Vahter M, Hökfelt T, Johnson JA, Johnson DA, Ceccatelli S (2007) Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci 97(2):428–437
Bromley-Brits K, Deng Y, Song W (2011) Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J Vis Exp 53
Seibenhener ML, Wooten MW (2012) Isolation and culture of hippocampal neurons from prenatal mice. J Vis Exp. 26(65)
Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E (2009) Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117(7):1131–1138
Pan M, Zhang C (2013) Stimulatory effect of gonadal hormones on fetal rat hippocampal neural proliferation requires neurotrophin receptor activation in vitro. Neurosci Lett. 546:1–5
Bisen-Hersh EB, Farina M, Barbosa Jr F, Rocha JB, Aschner M (2014) Behavioral effects of developmental methylmercury drinking water exposure in rodents. J Trace Elem Med Biol 28(2):117–124
Lilja AM, Malmsten L, Röjdner J, Voytenko L, Verkhratsky A, Ögren SO, Nordberg A, Marutle A (2015) Neural stem cell transplant-induced effect on neurogenesis and cognition in Alzheimer Tg2576 mice is inhibited by concomitant treatment with amyloid-lowering or cholinergic α7 nicotinic receptor drugs. Neural Plast. 2015:370432
Malfa GA, Tomasello B, Sinatra F, Villaggio G, Amenta F, Avola R, Renis M (2014) “Reactive” response evaluation of primary human astrocytes after methylmercury exposure. J Neurosci Res 92(1):95–103
Ceccatelli S, Bose R, Edoff K, Onishchenko N, Spulber S (2013) Long-lasting neurotoxic effects of exposure to methylmercury during development. J Intern Med 273(5):490–497
Bose R, Onishchenko N, Edoff K, Janson Lang AM, Ceccatelli S (2012) Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol Sci 130(2):383–390
Monnet-Tschudi F, Zurich MG, Boschat C, Corbaz A, Honegger P (2006) Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health 21(2):105–117
Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM (1996) Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following long term subclinical methylmercury exposure. Neurotoxicology 17(1):127–138
Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM (2007) Neural stem cells improve memory in an inducible mouse model of neuronal loss. The Joural of Neuroscience 27(44):11925–11933
Faustman EM, Ponce RA, Ou YC, Mendoza MA, Lewandowski T, Kavanagh T (2002) Investigations of methylmercury-induced alterations in neurogenesis. Environ Health Perspect 110(Suppl 5):859–864
Berninger B (2010) Making neurons from mature glia: a far-fetched dream? Neuropharmacology 58(6):894–902
Mori T, Buffo A, Götz M (2005) The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol 69:67–99
Chang JY, Tsai PF (2009) IL-6 release from mouse glia caused by MeHg requires cytosolic phospholipase A2 activation. Neurosci Lett 461(2):85–89
Jebbett NJ, Hamilton JW, Rand MD, Eckenstein F (2013) Low level methylmercury enhances CNTF-evoked STAT3 signaling and glial differentiation in cultured cortical progenitor cells. Neurotoxicology 38:91–100
Hagemann TL, Paylor R, Messing A (2013) Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J Neurosci. 33(47):18698–18706
Ben Haim L, Carrillo-de Sauvage MA, Ceyzériat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 81060231, 81160338, 30960110, 31360238) and Ningxia Universities Key Project (NGY2011039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experiments were conducted in accordance with the rules of the National Committee on Care and Use of Experimental Animal Resources and approved by the Animal Ethics Committee of Ningxia Medical University.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tian, J., Luo, Y., Chen, W. et al. MeHg Suppressed Neuronal Potency of Hippocampal NSCs Contributing to the Puberal Spatial Memory Deficits. Biol Trace Elem Res 172, 424–436 (2016). https://doi.org/10.1007/s12011-015-0609-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0609-8