Abstract
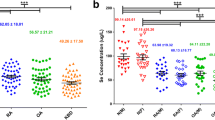
BCL2-antagonist/killer 1 (BAK1) and apoptotic peptidase activating factor 1 (APAF1) are significant genes in apoptosis signalling pathway of Kashin–Beck disease (KBD). We aimed to verify the protein expression levels of BAK1 and APAF1 in the cartilage and chondrocytes of patients with KBD. Additionally, we explored the relationship between the levels of these proteins and selenium concentration. Chondrocytes was cultured and treated with sodium selenite in vitro. Immunohistochemistry and Western blotting were used to verify the expression levels of BAK1 and APAF1. Compared with the control samples, APAF1 was upregulated and BAK1 was downregulated in the cartilage and chondrocytes of KBD patients. APAF1 expression was higher in the middle and deep zone in the KBD cartilage. APAF1 levels decreased gradually with the increasing selenium concentration (0.05, 0.10 and 0.25 mg/L). BAK1 expression in the 0.25 mg/L selenium group was lower than that of the control group. Different selenium concentrations had varying effects on BAK1 and APAF1 levels. APAF1 may play an important role in the pathogenesis of KBD. APAF1-related apoptosis was more pronounced in the middle and deep zones of the KBD cartilage. APAF may represent a potentially novel molecular target, which may be a biomarker of the role of selenium on the prevention and treatment of KBD. The role of BAK1 in the pathogenesis of KBD requires further study.






Similar content being viewed by others
References
Wang S, Duan C, Zhang F, Ma W, Guo X (2013) Regulatory gene networks and signaling pathways from primary osteoarthritis and Kashin-Beck disease, an endemic osteoarthritis, identified by three analysis software. Gene 1(512):89–96. doi:10.1016/j.gene.2012.10.006
Schepman K, Engelbert RH, Visser MM, Yu C, de Vos R (2011) Kashin Beck disease: more than just osteoarthrosis: a cross-sectional study regarding the influence of body function-structures and activities on level of participation. Int Orthop 5(35):767–776. doi:10.1007/s00264-010-1043-3
Yao Y, Pei F, Kang P (2011) Selenium, iodine, and the relation with Kashin-Beck disease. Nutrition 11-12(27):1095–1100. doi:10.1016/j.nut.2011.03.002
Zhao ZJ, Li Q, Yang PZ, Wang H, Kong LC, Wang LH, Sun LY (2013) Selenium: a protective factor for Kaschin-Beck disease in Qing-Tibet Plateau. Biol Trace Elem Res 1-3(153):1–4. doi:10.1007/s12011-013-9686-8
Wang J, Li H, Li Y, Yu J, Yang L, Feng F, Chen Z (2013) Speciation, distribution, and bioavailability of soil selenium in the Tibetan plateau Kashin-Beck disease area—a case study in Songpan County, Sichuan Province, China. Biol Trace Elem Res 1-3(156):367–375. doi:10.1007/s12011-013-9822-5
Moreno-Reyes R, Mathieu F, Boelaert M, Begaux F, Suetens C, Rivera MT, Neve J, Perlmutter N, Vanderpas J (2003) Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am J Clin Nutr 1(78):137–144
Meng F, Fu Y , Wang L (2001) The effect evaluation of the prevention of Kashin-Beck disease by the change of food and selenium supplementary. Chin J Ctrl Endem Dis 3 (26):188-190
Wang HY, Chen Z (2008) The paired study on the relationship between KBD and Selenium in hair. Chin J Ctrl Endem Dis 1(23):59–60
Yang J, Wang T, Wu C, Liu C (2010) Selenium level surveillance for the year 2007 of Keshan disease in endemic areas and analysis on surveillance results between 2003 and 2007. Biol Trace Elem Res 1-3(138):53–59. doi:10.1007/s12011-010-8609-1
Xiong YM, Mo XY, Zou XZ, Song RX, Sun WY, Lu W, Chen Q, Yu YX, Zang WJ (2010) Association study between polymorphisms in selenoprotein genes and susceptibility to Kashin-Beck disease. Osteoarthr Cartil 6(18):817–824. doi:10.1016/j.joca.2010.02.004
Du XH, Dai XX, Xia SR, Zou XZ, Yan SW, Mo XY, Lu BG, Xiong YM (2012) SNP and mRNA expression for glutathione peroxidase 4 in Kashin-Beck disease. Br J Nutr 2(107):164–169. doi:10.1017/S0007114511002704
Downey CM, Horton CR, Carlson BA, Parsons TE, Hatfield DL, Hallgrimsson B, Jirik FR (2009) Osteo-chondroprogenitor-specific deletion of the selenocysteine tRNA gene, Trsp, leads to chondronecrosis and abnormal skeletal development: a putative model for Kashin-Beck disease. PLoS Genet 8(5):e1000616. doi:10.1371/journal.pgen.1000616
Moreno-Reyes R, Egrise D, Neve J, Pasteels JL, Schoutens A (2001) Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res 8(16):1556–1563. doi:10.1359/jbmr.2001.16.8.1556
Wang S, Guo X, Wang W, Wang S (2012) Genome-wide study identifies the regulatory gene networks and signaling pathways from chondrocyte and peripheral blood monocyte of Kashin-Beck disease. Genes Cells 8(17):619–632. doi:10.1111/j.1365-2443.2012.01620.x
Liu JT, Guo X, Ma WJ, Zhang YG, Xu P, Yao JF, Bai YD (2010) Mitochondrial function is altered in articular chondrocytes of an endemic osteoarthritis, Kashin-Beck disease. Osteoarthr Cartil 9(18):1218–1226. doi:10.1016/j.joca.2010.07.003
Zheng J, Wu C, Ma W, Zhang Y, Hou T, Xu H, Wu S, Yao X, Guo X (2013) Abnormal expression of chondroitin sulphate N-acetylgalactosaminyltransferase 1 and hapln-1 in cartilage with Kashin-Beck disease and primary osteoarthritis. Int Orthop 10(37):2051–2059. doi:10.1007/s00264-013-1937-y
Gogada R, Prabhu V, Amadori M, Scott R, Hashmi S, Chandra D (2011) Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. J Biol Chem 33(286):28749–28760. doi:10.1074/jbc.M110.202440
Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA (2008) E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol 21(82):10408–10417. doi:10.1128/JVI.00902-08
Chen M, Huang L, Shabier Z, Wang J (2007) Regulation of the lifespan in dendritic cell subsets. Mol Immunol 10(44):2558–2565. doi:10.1016/j.molimm.2006.12.020
Radetzki S, Kohne CH, von Haefen C, Gillissen B, Sturm I, Dorken B, Daniel PT (2002) The apoptosis promoting Bcl-2 homologues Bak and Nbk/Bik overcome drug resistance in Mdr-1-negative and Mdr-1-overexpressing breast cancer cell lines. Oncogene 2(21):227–238. doi:10.1038/sj.onc.1205010
Kuwano K, Yoshimi M, Maeyama T, Hamada N, Yamada M, Nakanishi Y (2005) Apoptosis signaling pathways in lung diseases. Med Chem 1(1):49–56
Herr I, Debatin KM (2001) Cellular stress response and apoptosis in cancer therapy. Blood 9(98):2603–2614
Kumamoto H, Ooya K (2005) Detection of mitochondria-mediated apoptosis signaling molecules in ameloblastomas. J Oral Pathol Med 9(34):565–572. doi:10.1111/j.1600-0714.2005.00354.x
Pasteels JL, Liu FD, Hinsenkamp M, Rooze M, Mathieu F, Perlmutter N (2001) Histology of Kashin-Beck lesions. Int Orthop 3(25):151–153
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81402638, 81472924 and 81472925).
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Duan, C., Zhang, F. et al. The Roles of the Interaction of BCL2-Antagonist/Killer 1, Apoptotic Peptidase Activating Factor 1 and Selenium in the Pathogenesis of Kashin–Beck Disease. Biol Trace Elem Res 170, 17–24 (2016). https://doi.org/10.1007/s12011-015-0424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0424-2